Rising Incidence of Infectious Diseases
The rising incidence of infectious diseases in China is a critical factor influencing the recombinant vaccines market. With outbreaks of diseases such as influenza and hepatitis, there is an urgent need for effective vaccination strategies. The World Health Organization (WHO) has reported an increase in vaccine-preventable diseases, prompting public health authorities to prioritize vaccination programs. This heightened awareness is likely to drive demand for recombinant vaccines, as they offer targeted solutions for disease prevention. The market is expected to grow by approximately 8% annually, reflecting the urgent need for innovative vaccine options to combat these health challenges.
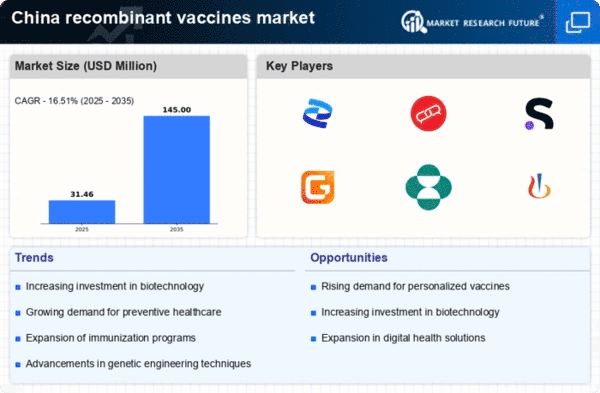
Growing Demand for Preventive Healthcare
The increasing focus on preventive healthcare in China is driving the recombinant vaccines market. As the population becomes more health-conscious, there is a notable shift towards vaccination as a proactive measure against infectious diseases. This trend is supported by government initiatives promoting vaccination programs, which aim to enhance public health outcomes. In 2025, the market is projected to reach approximately $2 billion, reflecting a compound annual growth rate (CAGR) of around 10% over the next five years. The emphasis on preventive measures is likely to bolster the demand for innovative vaccine solutions, thereby expanding the recombinant vaccines market.
Regulatory Support for Vaccine Development
The regulatory landscape in China is evolving to support the recombinant vaccines market. Recent reforms have streamlined the approval process for new vaccines, encouraging innovation and expediting market entry. The National Medical Products Administration (NMPA) has implemented measures to enhance the efficiency of clinical trials and product approvals. This regulatory support is crucial for fostering a conducive environment for vaccine development, potentially leading to a surge in new recombinant vaccines. As a result, the market is likely to experience accelerated growth, with an estimated increase in market value by 15% over the next five years.
Advancements in Biopharmaceutical Manufacturing
Technological innovations in biopharmaceutical manufacturing are significantly impacting the recombinant vaccines market. The adoption of advanced production techniques, such as cell culture and fermentation processes, enhances the efficiency and yield of vaccine production. In China, the biopharmaceutical sector is expected to grow at a CAGR of 12% from 2025 to 2030, driven by these advancements. This growth is likely to facilitate the development of more effective recombinant vaccines, addressing a wider range of diseases. Consequently, the recombinant vaccines market is poised for expansion as manufacturers leverage these technologies to meet increasing demand.
Increased Investment in Research and Development
Investment in research and development (R&D) is a key driver for the recombinant vaccines market in China. Both public and private sectors are allocating substantial resources to advance vaccine technologies and discover new applications. In 2025, R&D spending in the biopharmaceutical sector is projected to exceed $5 billion, reflecting a growing commitment to innovation. This influx of funding is expected to facilitate breakthroughs in recombinant vaccine formulations, enhancing their efficacy and safety profiles. Consequently, the recombinant vaccines market is likely to benefit from a robust pipeline of new products, catering to diverse health needs.