Government Initiatives and Funding
Government initiatives and funding play a crucial role in the growth of the recombinant vaccines market. In the US, federal and state governments are increasingly investing in vaccine research and development to combat infectious diseases. For example, the National Institutes of Health (NIH) allocates substantial funding for vaccine-related research, which supports the advancement of recombinant technologies. This financial backing is essential for fostering innovation and ensuring that the recombinant vaccines market remains competitive. Furthermore, public-private partnerships are emerging, facilitating collaboration between government entities and private companies. Such initiatives are likely to enhance the development pipeline of recombinant vaccines, ultimately benefiting public health.
Innovations in Vaccine Development
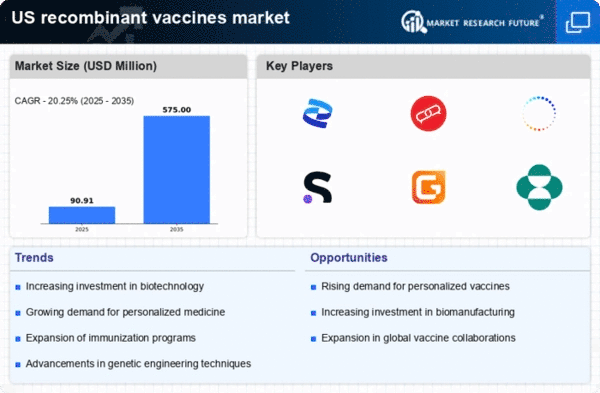
Innovative technologies in vaccine development are significantly impacting the recombinant vaccines market. Advances in genetic engineering and biotechnology have enabled the creation of more effective and safer vaccines. For instance, the use of recombinant DNA technology allows for the production of vaccines that can elicit stronger immune responses. The recombinant vaccines market is projected to grow at a CAGR of 8.5% from 2025 to 2030, driven by these innovations. Additionally, the ability to rapidly develop vaccines in response to emerging infectious diseases enhances the attractiveness of recombinant vaccines. This innovation not only addresses public health needs but also positions the recombinant vaccines market as a leader in the biopharmaceutical sector.
Rising Demand for Preventive Healthcare
The increasing emphasis on preventive healthcare is driving the recombinant vaccines market. As healthcare systems shift focus from treatment to prevention, the demand for vaccines that can effectively prevent diseases is surging. This trend is particularly evident in the US, where healthcare expenditures are projected to reach $4.3 trillion by 2025. The recombinant vaccines market is poised to benefit from this shift, as these vaccines offer enhanced efficacy and safety profiles. Moreover, the growing awareness of vaccine-preventable diseases among the population is likely to further stimulate demand. As a result, healthcare providers are increasingly incorporating recombinant vaccines into their immunization programs, thereby expanding their market presence.
Growing Investment in Biopharmaceuticals
The increasing investment in biopharmaceuticals is positively influencing the recombinant vaccines market. As the biopharmaceutical sector expands, there is a corresponding rise in funding for vaccine development, particularly recombinant vaccines. The US biopharmaceutical market is expected to reach $500 billion by 2025, with a significant portion allocated to vaccine research. This investment is crucial for advancing technologies that enhance vaccine efficacy and safety. Additionally, the recombinant vaccines market stands to benefit from collaborations between biopharmaceutical companies and research institutions, fostering innovation and accelerating the development of new vaccines. This trend indicates a robust future for the recombinant vaccines market as it aligns with the broader growth of the biopharmaceutical industry.
Increasing Incidence of Infectious Diseases
The rising incidence of infectious diseases is a significant driver for the recombinant vaccines market. With the emergence of new pathogens and the resurgence of previously controlled diseases, there is an urgent need for effective vaccination strategies. The Centers for Disease Control and Prevention (CDC) reports that vaccine-preventable diseases continue to pose a threat to public health in the US. This situation creates a favorable environment for the recombinant vaccines market, as these vaccines can be tailored to target specific pathogens. The urgency to develop vaccines against diseases such as influenza, Zika, and others is likely to propel market growth, as healthcare providers seek effective solutions to protect populations.