Advancements in Stem Cell Research
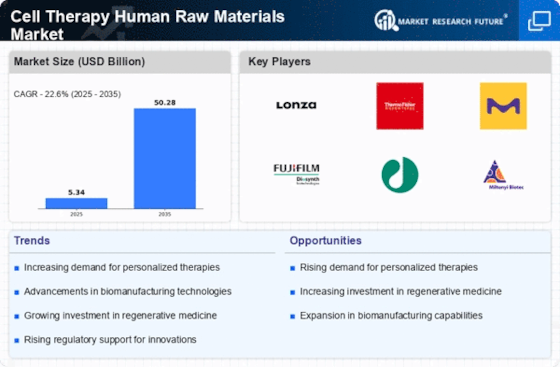
Recent advancements in stem cell research are propelling the Cell Therapy Human Raw Materials Market forward. Innovations in understanding stem cell biology and their potential applications in regenerative medicine have opened new avenues for treatment. Research indicates that stem cells can differentiate into various cell types, offering promising solutions for conditions previously deemed untreatable. The market for stem cell-based therapies is projected to grow substantially, with estimates suggesting a compound annual growth rate of over 20% in the coming years. This growth is likely to be fueled by increased investment in research and development, as well as collaborations between academic institutions and biotechnology firms. Consequently, the Cell Therapy Human Raw Materials Market stands to benefit from these scientific breakthroughs, enhancing the availability and efficacy of cell-based therapies.
Regulatory Support for Cell Therapies
Regulatory bodies are increasingly providing support for the development and commercialization of cell therapies, which is a crucial driver for the Cell Therapy Human Raw Materials Market. Streamlined approval processes and clear guidelines are encouraging companies to invest in cell-based therapies. Recent initiatives by regulatory agencies aim to facilitate faster access to innovative treatments, thereby enhancing patient care. This supportive regulatory environment is likely to stimulate market growth, as companies can navigate the approval landscape more efficiently. Additionally, the establishment of specialized regulatory frameworks for advanced therapies is expected to further bolster the Cell Therapy Human Raw Materials Market. As a result, the alignment of regulatory policies with industry needs may lead to an increase in the availability of cell therapies, ultimately benefiting patients worldwide.
Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases such as cancer, diabetes, and autoimmune disorders is a primary driver for the Cell Therapy Human Raw Materials Market. As these conditions become more prevalent, the demand for innovative treatment options rises. Cell therapies, which utilize human raw materials, are gaining traction as effective solutions. According to recent estimates, the global burden of chronic diseases is expected to reach unprecedented levels, necessitating advanced therapeutic interventions. This trend indicates a growing market for cell therapy products, as healthcare providers seek to incorporate these therapies into treatment protocols. The Cell Therapy Human Raw Materials Market is thus positioned to expand significantly, driven by the urgent need for effective therapies that address these complex health challenges.
Growing Investment in Biopharmaceuticals
The surge in investment within the biopharmaceutical sector is significantly influencing the Cell Therapy Human Raw Materials Market. As pharmaceutical companies recognize the potential of cell therapies, funding for research and development has increased markedly. Reports indicate that investments in biopharmaceuticals have reached record levels, with billions allocated to innovative therapies, including cell-based treatments. This influx of capital is likely to accelerate the development of new products and technologies, thereby expanding the market for human raw materials used in cell therapies. Furthermore, partnerships between biopharmaceutical companies and research institutions are fostering innovation, which may lead to the introduction of novel therapies. The Cell Therapy Human Raw Materials Market is thus poised for growth, driven by this robust financial backing and the ongoing quest for effective treatment solutions.
Increased Focus on Regenerative Medicine
The heightened focus on regenerative medicine is driving the Cell Therapy Human Raw Materials Market. As the medical community seeks to develop therapies that restore or replace damaged tissues and organs, cell therapies are emerging as a viable solution. The regenerative medicine market is projected to grow significantly, with estimates suggesting a valuation of over 100 billion in the next few years. This growth is attributed to advancements in technology and a better understanding of cellular mechanisms. The Cell Therapy Human Raw Materials Market is likely to benefit from this trend, as the demand for high-quality human raw materials increases to support the development of regenerative therapies. Consequently, the intersection of regenerative medicine and cell therapy is expected to create new opportunities for innovation and market expansion.