Market Share
Cell Banking Outsourcing Market Share Analysis
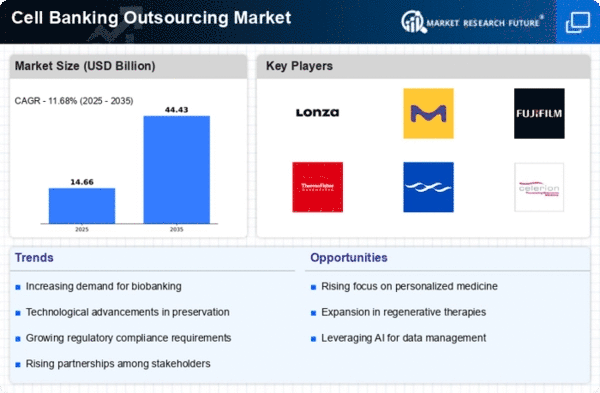
The Cell Banking Outsourcing market is a dynamic sector in the biopharmaceutical enterprise, witnessing widespread growth. Market percentage positioning strategies play a vital role in determining the achievement of organizations running in this space. To effectively position themselves inside the Cell Banking Outsourcing market, businesses want a comprehensive understanding of the industry's dynamics. Factors inclusive of growing demand for biologics, improvements in Cell remedy, and stringent regulatory requirements shape the panorama. Companies aiming to steady a sizable market percentage often adopt a strategy of specialization. The Cell Banking Outsourcing market is not restricted to a selected area. Successful groups often strategize for global expansion to tap into numerous markets. Establishing a presence in key regions permits them to attain a broader consumer base and adapt to various regulatory landscapes. Staying in advance within the market requires continuous technological innovation. Companies invest in modern technology for cell line improvement, garages, and retrieval. The integration of automation and synthetic intelligence enhances performance, attracting clients searching for present-day answers. Collaboration is a key method for market positioning. Companies forge strategic partnerships with biopharmaceutical companies, research institutions, and agreement production companies. These alliances now not only develop carrier offerings but also create synergies for mutual growth. Given the extremely regulated nature of the biopharmaceutical industry, compliance with international first-class requirements is paramount. Companies that prioritize and exhibit sturdy, great warranty measures role themselves as dependable partners for clients looking for compliance and risk mitigation. Recognizing the numerous needs of customers, a hit corporation within the Cell Banking Outsourcing market provides customizable offerings. Tailoring solutions to suit precise requirements, whether in phrases of Cell type, scale, or procedure, complements client satisfaction and loyalty. Building and maintaining a sturdy emblem popularity is essential in a market where trust is paramount. Companies that continually supply on promises, adhere to timelines and show transparency in their methods are likely to attract and hold faithful shoppers. Regulatory frameworks governing the biopharmaceutical industry are concerned with consistent modifications. Companies positioning themselves for achievement inside the Cell Banking Outsourcing market invest in regulatory intelligence and demonstrate adaptability to evolving compliance requirements. Establishing concept leadership via actively participating in industry meetings, publishing studies, and imparting instructional assets positions agencies as experts in the area. This now not only draws clients but also contributes to shaping enterprise standards.

















Leave a Comment