Market Analysis
In-depth Analysis of Cell Banking Outsourcing Market Industry Landscape
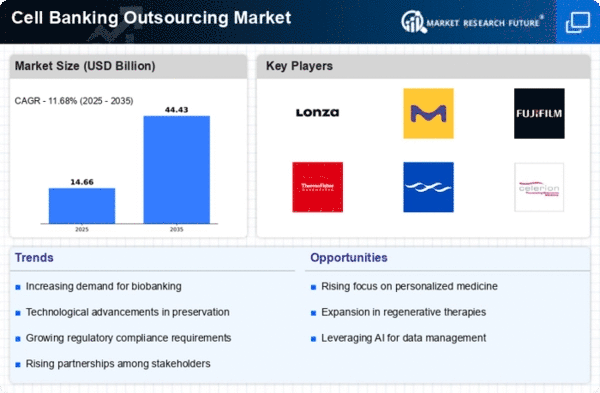
The Cell Banking Outsourcing market has witnessed a vast increase in recent years, pushed by the growing demand for Cell-primarily based treatment plans and biopharmaceuticals. As organizations focus on their middle talents, outsourcing cell banking offerings has become a strategic desire, mainly due to a dynamic and competitive market panorama. The escalating need for revolutionary cell-based total treatment plans, including stem cell cures and gene remedies, has fueled the demand for reliable and green cell banking offerings. Outsourcing permits businesses to leverage specialized know-how, riding advancements in the subject and assembly the burgeoning international demand for customized medicine. Biopharmaceutical enterprises play a pivotal role in propelling the cell banking outsourcing market. As biotech agencies expand their portfolios, outsourcing cell banking services permits them to streamline operations, reduce expenses, and boost the development and commercialization of novel therapeutics. Ongoing technological advancements in Cell culture and preservation strategies have improved the talents of Cell banking service providers. Outsourcing permits groups to undertake the latest technology without good-sized capital investments, riding efficiency and exceptional in the Cell banking process. Cost considerations and aid optimization are key drivers for outsourcing in the Cell banking market. By outsourcing non-core sports, companies can allocate resources strategically that specialize in research, improvement, and commercialization efforts, even leveraging outside expertise for cell banking procedures. The emergence of specialized contract research organizations (CROs) committed to Cell banking offerings has contributed to the market's dynamics. CROs offer end-to-stop answers, from Cell line development to grasp cell bank manufacturing, presenting a one-prevent store for businesses trying to outsource their cell banking wishes. Risk mitigation is an important consideration for agencies engaged in the improvement of biopharmaceuticals. Outsourcing cell banking services allows for threat-sharing and the transfer of certain operational risks to skilled carrier companies, fostering an extra resilient and adaptive enterprise version. Looking ahead, the cell banking outsourcing market is anticipated to witness an increased increase, pushed by advancements in cell-based healing procedures, increasing collaborations, and the continuing evolution of regulatory frameworks. The market dynamics could be shaped by way of the interaction of technological innovation, regulatory compliance, and the strategic outsourcing decisions of biopharmaceutical groups.

















Leave a Comment