Market Share
Biomarker Technologies Market Share Analysis
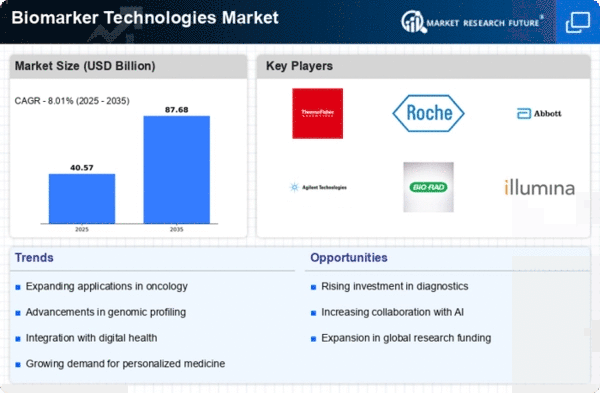
The market dynamics of Biomarker Technologies unfold within a landscape characterized by groundbreaking scientific discoveries, a growing focus on personalized medicine, and advancements in diagnostic capabilities. Biomarkers, measurable indicators of biological processes or conditions, have become integral in various aspects of healthcare, including disease diagnosis, prognosis, and treatment monitoring. One key driver of the market is the expanding role of biomarkers in precision medicine, where treatment approaches are tailored to an individual's unique biological characteristics. As the understanding of the molecular basis of diseases deepens, the demand for innovative biomarker technologies rises, shaping the market dynamics significantly.
Technological innovation plays a pivotal role in shaping the market dynamics of Biomarker Technologies. Advances in genomics, proteomics, and other high-throughput technologies have revolutionized the identification and analysis of biomarkers. Next-generation sequencing, mass spectrometry, and other cutting-edge platforms enable the rapid and comprehensive profiling of biomolecules, contributing to the discovery of novel biomarkers associated with various diseases. These technological advancements empower healthcare professionals to make more accurate and timely diagnoses, stratify patients for targeted therapies, and monitor treatment responses, thus enhancing the overall efficiency and effectiveness of medical interventions.
Collaborations and partnerships among research institutions, biotechnology companies, and healthcare organizations contribute to the dynamic landscape of the Biomarker Technologies market. These alliances facilitate the translation of research findings into practical applications, drive the development of standardized biomarker assays, and promote knowledge exchange within the scientific community. The collaborative efforts in biomarker research pave the way for the discovery of new biomarkers, validation of existing ones, and their integration into clinical practice, fostering innovation and advancements in healthcare.
The pharmaceutical industry's engagement in biomarker research and drug development is a significant factor shaping market dynamics. Biomarkers play a crucial role in drug discovery and development, aiding in patient stratification, target identification, and assessment of treatment responses. The pharmaceutical sector invests in biomarker-driven clinical trials, seeking to identify predictive and prognostic biomarkers that guide the development and successful deployment of targeted therapies. The integration of biomarkers into drug development processes not only accelerates the path to market but also contributes to more efficient and cost-effective clinical trials.
Regulatory frameworks and standards also influence the market dynamics of Biomarker Technologies. Regulatory agencies play a crucial role in establishing guidelines for the validation, qualification, and utilization of biomarkers in clinical settings. Compliance with regulatory requirements ensures the reliability and reproducibility of biomarker assays, instilling confidence in healthcare professionals, patients, and industry stakeholders. The regulatory landscape provides a framework for the responsible development and deployment of biomarker technologies, safeguarding patient safety and promoting the adoption of reliable diagnostic tools.
Economic factors, including healthcare spending and reimbursement policies, contribute to the market dynamics of Biomarker Technologies. The economic feasibility of biomarker testing and the availability of reimbursement options influence their accessibility and adoption within healthcare systems. As stakeholders seek cost-effective solutions that align with budgetary constraints, the market responds by striving to deliver value-based biomarker technologies for improved patient outcomes.

















Leave a Comment