-
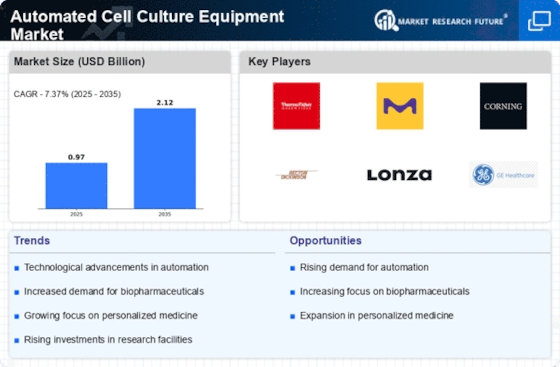
Executive Summary
-
MARKET SYNOPSIS 18
-
Market Introduction
-
DEFINITION 19
-
SCOPE OF THE STUDY 19
-
RESEARCH OBJECTIVE 19
-
MARKET STRUCTURE 20
-
Research Methodology
-
RESEARCH PROCESS
-
21
-
PRIMARY RESEARCH 22
-
SECONDARY RESEARCH 23
-
MARKET SIZE
-
ESTIMATION 23
-
FORECAST MODEL 25
-
LIST OF ASSUMPTIONS 26
-
4
-
MARKET DYNAMICS
-
OVERVIEW 27
-
DRIVERS 28
- GROWING RESEARCH
- RISE IN
- INCREASING PREVALENCE OF CANCER
-
AND DEVELOPMENT INITIATIVES IN THE PHARMACEUTICAL INDUSTRY 28
-
ADOPTION OF LIQUID HANDLING SYSTEMS 28
-
AND OTHER CHRONIC DISEASES 29
-
RESTRAINTS 30
- HIGH MAINTAINANCE
-
COST 30
-
OPPORTUNITIES 31
- RISING FOCUS ON REGENERATIVE MEDICINE
-
31
-
MARKET FACTOR ANALYSIS
-
PORTER’S FIVE FORCES MODEL 32
- BARGAINING POWER OF SUPPLIERS 33
- BARGAINING POWER OF BUYERS 33
- THREAT OF NEW ENTRANTS 33
- THREAT OF SUBSTITUTES 33
-
5.1.5
-
INTENSITY OF RIVALRY 33
-
VALUE CHAIN ANALYSIS 34
- R&D AND
- MANUFACTURING 35
- DISTRIBUTION & SALES 35
- POST-SALES MONITORING 35
-
DESIGNING 35
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY LAB AUTOMATION TYPE
-
OVERVIEW 36
-
MODULAR AUTOMATION 38
-
6.3
-
WHOLE LAB AUTOMATION 38
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT
-
TYPE
-
OVERVIEW 39
-
CONSUMABLES 42
-
EQUIPMENT 42
-
7.4
-
SOFTWARE 43
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
8.1
-
OVERVIEW 44
-
BIOPHARMACEUTICAL PRODUCTION 46
-
TISSUE ENGINEERING
-
47
-
VACCINE PRODUCTION 47
-
DRUG SCREENING AND DEVELOPMENT 48
-
GENE THERAPY & REGENERATIVE MEDICINE 48
-
STEM CELL THERAPY 49
-
TOXICITY TESTING 49
-
DIAGNOSTICS 50
-
GLOBAL AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, END USER
-
OVERVIEW 51
-
PHARMACEUTICAL & BIOTECHNOLOGY
-
COMPANIES 53
-
RESEARCH AND ACADEMIC INSTITUTES 53
-
HOSPITALS AND
-
DIAGNOSTIC LABORATORIES 54
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY REGION
-
OVERVIEW 55
-
AMERICAS 57
-
AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
NORTH AMERICA 61
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION
-
TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
AUTOMATED
-
US 64
-
CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY LAB AUTOMATION TYPE
-
CANADA 67
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT
-
TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
AMERICA 70
-
LATIN
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY APPLICATION
-
EUROPE 74
-
AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
WESTERN EUROPE 78
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB
-
AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
82
-
GERMANY
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY APPLICATION
-
AUTOMATED CELL CULTURE EQUIPMENT
-
UK 85
-
MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY
-
PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
10.3.1.3
-
FRANCE 88
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY APPLICATION
-
AUTOMATED CELL CULTURE
-
ITALY 91
-
EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
SPAIN 94
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION
-
TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
97
-
REST OF WESTERN EUROPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY APPLICATION
-
AUTOMATED CELL CULTURE
-
EASTERN EUROPE 100
-
EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
ASIA-PACIFIC 103
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION
-
TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
AUTOMATED
-
JAPAN 107
-
CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY LAB AUTOMATION TYPE
-
CHINA 110
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT
-
TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
113
-
AUSTRALIA
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY APPLICATION
-
AUTOMATED CELL CULTURE EQUIPMENT
-
INDIA 116
-
MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY
-
PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
10.4.5
-
SOUTH KOREA 119
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION
-
TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
REST OF ASIA-PACIFIC 122
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY APPLICATION
-
MIDDLE EAST & AFRICA 126
-
AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
MIDDLE EAST 130
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION
-
TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
AUTOMATED
-
AFRICA 133
-
CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE
-
AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY PRODUCT TYPE
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION
-
COMPETITIVE LANDSCAPE
-
INTRODUCTION 136
-
11.2
-
COMPETITIVE ANALYSIS 136
-
COMPANY PROFILES
-
TECAN TRADING AG 137
- COMPANY OVERVIEW 137
- FINANCIAL OVERVIEW 138
- PRODUCTS/SERVICES
- KEY DEVELOPMENTS 140
- SWOT ANALYSIS 141
-
OFFERED 139
-
12.1.6
-
KEY STRATEGIES 141
-
SIEMENS 142
- COMPANY OVERVIEW 142
- PRODUCTS/SERVICES OFFERED 143
- KEY
- SWOT ANALYSIS 144
- KEY STRATEGIES 144
-
12.2.2
-
FINANCIAL OVERVIEW 142
-
DEVELOPMENTS 143
-
HITACHI, LTD 145
- COMPANY OVERVIEW 145
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED 146
- KEY DEVELOPMENTS 146
- SWOT ANALYSIS 147
- KEY STRATEGIES 147
-
145
-
THERMO FISHER
- COMPANY OVERVIEW 148
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED 149
- KEY DEVELOPMENTS 149
- SWOT ANALYSIS 150
- KEY STRATEGIES 150
-
SCIENTIFIC INC. 148
-
148
-
SARTORIUS AG
- COMPANY OVERVIEW 151
- FINANCIAL OVERVIEW 151
- KEY DEVELOPMENTS 152
- SWOT
- KEY STRATEGIES 153
-
151
-
12.5.3
-
PRODUCTS/SERVICES OFFERED 152
-
ANALYSIS 153
-
HAMILTON COMPANY 154
- FINANCIAL OVERVIEW 154
- PRODUCTS/SERVICES
- KEY DEVELOPMENTS 154
- SWOT ANALYSIS 155
-
12.6.1
-
COMPANY OVERVIEW 154
-
OFFERED 154
-
12.6.6
-
KEY STRATEGIES 155
-
BECKMAN COULTER, INC. 156
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW 156
- PRODUCTS/SERVICES OFFERED 157
- KEY DEVELOPMENTS 157
- SWOT ANALYSIS 158
- KEY STRATEGIES
-
156
-
158
-
F. HOFFMANN-LA ROCHE LTD 159
- COMPANY OVERVIEW 159
- PRODUCTS/SERVICES OFFERED 160
- KEY
- SWOT ANALYSIS 161
- KEY STRATEGIES 161
-
12.8.2
-
FINANCIAL OVERVIEW 160
-
DEVELOPMENTS 161
-
PERKINELMER, INC. 162
- COMPANY OVERVIEW 162
- FINANCIAL
- PRODUCTS/SERVICES OFFERED 163
- KEY DEVELOPMENTS
- SWOT ANALYSIS 164
- KEY STRATEGIES 164
-
OVERVIEW 162
-
163
-
ANTON
- COMPANY OVERVIEW 165
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED 165
- KEY DEVELOPMENTS 165
- SWOT ANALYSIS 166
- KEY STRATEGIES 166
-
PAAR GMBH 165
-
165
-
APPENDIX
-
REFERENCES 167
-
RELATED REPORTS 167
-
List of Tables
-
TABLE
-
LIST OF ASSUMPTIONS 26
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION) 37
-
GLOBAL AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET FOR MODULAR AUTOMATION, BY REGION, 2020–2027
-
(USD MILLION) 38
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR
-
WHOLE LAB AUTOMATION, BY REGION, 2020–2027
-
(USD MILLION) 38
-
TABLE
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE, 2020–2027
-
(USD MILLION) 40
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR
-
CONSUMABLES, BY TYPE, 2020–2027 (USD MILLION) 40
-
GLOBAL AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE, 2020–2027 (USD MILLION)
-
41
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY
-
TYPE, 2020–2027 (USD MILLION) 41
-
GLOBAL AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET FOR CONSUMABLES, BY REGION, 2020–2027 (USD MILLION) 42
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR EQUIPMENT, BY REGION,
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET FOR SOFTWARE, BY REGION, 2020–2027 (USD MILLION) 43
-
GLOBAL
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
46
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR BIOPHARMACEUTICAL
-
PRODUCTION, BY REGION, 2020–2027
-
(USD MILLION) 46
-
GLOBAL
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR TISSUE ENGINEERING, BY REGION, 2020–2027
-
(USD MILLION) 47
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR
-
VACCINE PRODUCTION, BY REGION, 2020–2027 (USD MILLION) 47
-
GLOBAL
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR DRUG SCREENING AND DEVELOPMENT, BY REGION,
-
(USD MILLION) 48
-
GLOBAL AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET FOR GENE THERAPY & REGENERATIVE MEDICINE, BY REGION,
-
(USD MILLION) 48
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR
-
STEM CELL THERAPY, BY REGION, 2020–2027 (USD MILLION) 49
-
GLOBAL
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR TOXICITY TESTING, BY REGION, 2020–2027
-
(USD MILLION) 49
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR
-
DIAGNOSTICS, BY REGION, 2020–2027 (USD MILLION) 50
-
GLOBAL AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY END USER, 2020–2027 (USD MILLION) 52
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR PHARMACEUTICAL &
-
BIOTECHNOLOGY COMPANIES, BY REGION, 2020–2027 (USD MILLION) 53
-
TABLE
-
GLOBAL AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR RESEARCH AND ACADEMIC INSTITUTES,
-
BY REGION, 2020–2027 (USD MILLION) 53
-
GLOBAL AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET FOR HOSPITALS AND DIAGNOSTIC LABORATORIES, BY REGION,
-
AMERICAS: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY REGION, 2020–2027 (USD MILLION) 57
-
AMERICAS:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027
-
(USD MILLION) 58
-
AMERICAS: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY PRODUCT TYPE 2020–2027 (USD MILLION) 58
-
AMERICAS: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET FOR CONSUMABLES, BY TYPE, 2020–2027 (USD MILLION)
-
58
-
AMERICAS: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR EQUIPMENT,
-
BY TYPE, 2020–2027 (USD MILLION) 59
-
AMERICAS: AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET FOR SOFTWARE, BY TYPE, 2020–2027 (USD MILLION) 59
-
AMERICAS: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027
-
(USD MILLION) 60
-
AMERICAS: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY END USER 2020–2027 (USD MILLION) 60
-
NORTH AMERICA: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY COUNTRY, 2020–2027 (USD MILLION) 61
-
TABLE
-
NORTH AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE,
-
NORTH AMERICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 62
-
TABLE 36
-
NORTH AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR CONSUMABLES, BY TYPE,
-
NORTH AMERICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET FOR EQUIPMENT, BY TYPE, 2020–2027 (USD MILLION) 62
-
TABLE
-
NORTH AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR SOFTWARE, BY TYPE,
-
NORTH AMERICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION) 63
-
TABLE 40
-
NORTH AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027
-
(USD MILLION) 64
-
US: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB
-
AUTOMATION TYPE, 2020–2027 (USD MILLION) 64
-
US: AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 64
-
TABLE
-
US: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR CONSUMABLES, BY TYPE, 2020–2027
-
(USD MILLION) 65
-
US: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR EQUIPMENT,
-
BY TYPE, 2020–2027 (USD MILLION) 65
-
US: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET FOR SOFTWARE, BY TYPE, 2020–2027 (USD MILLION) 66
-
TABLE
-
US: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD
-
MILLION) 66
-
US: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER
-
CANADA: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION) 67
-
CANADA:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION)
-
67
-
CANADA: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR CONSUMABLES,
-
BY TYPE, 2020–2027 (USD MILLION) 68
-
CANADA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET FOR EQUIPMENT, BY TYPE, 2020–2027 (USD MILLION) 68
-
TABLE
-
CANADA: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR SOFTWARE, BY TYPE, 2020–2027
-
(USD MILLION) 69
-
CANADA: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION 2020–2027 (USD MILLION) 69
-
CANADA: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION) 70
-
TABLE
-
LATIN AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE,
-
LATIN AMERICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 70
-
TABLE 57
-
LATIN AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR CONSUMABLES, BY TYPE,
-
LATIN AMERICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET FOR EQUIPMENT, BY TYPE, 2020–2027 (USD MILLION) 71
-
TABLE
-
LATIN AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET FOR SOFTWARE, BY TYPE,
-
LATIN AMERICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION) 72
-
TABLE 61
-
LATIN AMERICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027
-
(USD MILLION) 73
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY REGION, 2020–2027 (USD MILLION) 74
-
EUROPE: AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION)
-
75
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION) 75
-
TABLE 66
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 76
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 76
-
EUROPE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION) 77
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027
-
(USD MILLION) 77
-
WESTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY COUNTRY, 2020–2027 (USD MILLION) 78
-
EUROPE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION)
-
79
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION) 79
-
TABLE 74
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 80
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 80
-
EUROPE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION) 81
-
EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027
-
(USD MILLION) 81
-
GERMANY: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION) 82
-
GERMANY:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION)
-
82
-
GERMANY: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES,
-
BY TYPE 2020–2027 (USD MILLION) 82
-
GERMANY: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027 (USD MILLION) 83
-
TABLE
-
GERMANY: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027
-
(USD MILLION) 83
-
GERMANY: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION 2020–2027 (USD MILLION) 84
-
GERMANY: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION) 84
-
TABLE
-
UK: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027
-
(USD MILLION) 85
-
UK: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT
-
TYPE 2020–2027 (USD MILLION) 85
-
UK: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION) 85
-
TABLE 88
-
UK: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 86
-
UK: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR
-
SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 86
-
UK: AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION) 87
-
TABLE
-
UK: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD
-
MILLION) 87
-
FRANCE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB
-
AUTOMATION TYPE, 2020–2027 (USD MILLION) 88
-
FRANCE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 88
-
FRANCE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES, BY
-
TYPE 2020–2027 (USD MILLION) 88
-
FRANCE: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027 (USD MILLION) 89
-
TABLE
-
FRANCE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027
-
(USD MILLION) 89
-
FRANCE: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION 2020–2027 (USD MILLION) 90
-
FRANCE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION) 90
-
TABLE
-
ITALY: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027
-
(USD MILLION) 91
-
ITALY: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY PRODUCT TYPE 2020–2027 (USD MILLION) 91
-
ITALY: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION)
-
91
-
ITALY: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT,
-
BY TYPE 2020–2027 (USD MILLION) 92
-
ITALY: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 92
-
TABLE
-
ITALY: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027
-
(USD MILLION) 93
-
ITALY: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY END USER 2020–2027 (USD MILLION) 93
-
SPAIN: AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION)
-
94
-
SPAIN: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE
-
SPAIN: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION) 94
-
TABLE 109
-
SPAIN: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 95
-
SPAIN: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 95
-
SPAIN: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION) 96
-
SPAIN: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027
-
(USD MILLION) 96
-
REST OF WESTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY LAB AUTOMATION TYPE, 2020–2027
-
(USD MILLION) 97
-
TABLE
-
REST OF WESTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT
-
TYPE 2020–2027 (USD MILLION) 97
-
REST OF WESTERN EUROPE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES, BY TYPE 2020–2027
-
(USD
-
MILLION) 97
-
REST OF WESTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 98
-
TABLE
-
REST OF WESTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE,
-
BY TYPE 2020–2027
-
(USD MILLION) 98
-
REST OF WESTERN EUROPE:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION)
-
99
-
REST OF WESTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY END USER 2020–2027 (USD MILLION) 99
-
EASTERN EUROPE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION)
-
100
-
EASTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT
-
TYPE 2020–2027 (USD MILLION) 100
-
EASTERN EUROPE: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION)
-
100
-
EASTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR
-
EQUIPMENT, BY TYPE 2020–2027 (USD MILLION) 101
-
EASTERN EUROPE:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027 (USD
-
MILLION) 101
-
EASTERN EUROPE: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION 2020–2027 (USD MILLION) 102
-
EASTERN EUROPE:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION)
-
102
-
ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY COUNTRY,
-
ASIA-PACIFIC: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION) 104
-
TABLE
-
ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027
-
(USD MILLION) 104
-
ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION) 105
-
TABLE 131
-
ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 105
-
ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 106
-
ASIA-PACIFIC:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION)
-
106
-
ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END
-
USER 2020–2027 (USD MILLION) 107
-
JAPAN: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION) 107
-
TABLE
-
JAPAN: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027
-
(USD MILLION) 107
-
JAPAN: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION) 108
-
JAPAN:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 108
-
JAPAN: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 109
-
JAPAN: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY APPLICATION 2020–2027 (USD MILLION) 109
-
JAPAN: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027
-
(USD MILLION) 110
-
CHINA: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY LAB AUTOMATION TYPE, 2020–2027 (USD MILLION) 110
-
CHINA:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION)
-
110
-
CHINA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES,
-
BY TYPE 2020–2027 (USD MILLION) 111
-
CHINA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027 (USD MILLION) 111
-
TABLE
-
CHINA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027
-
(USD MILLION) 112
-
CHINA: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION 2020–2027 (USD MILLION) 112
-
CHINA: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION) 113
-
AUSTRALIA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION
-
TYPE, 2020–2027 (USD MILLION) 113
-
AUSTRALIA: AUTOMATED CELL
-
CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 113
-
TABLE
-
AUSTRALIA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES, BY TYPE
-
AUSTRALIA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027 (USD MILLION) 114
-
TABLE
-
AUSTRALIA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027
-
(USD MILLION) 115
-
AUSTRALIA: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY APPLICATION 2020–2027 (USD MILLION) 115
-
AUSTRALIA: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION) 116
-
INDIA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE,
-
INDIA: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 117
-
INDIA:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES, BY TYPE 2020–2027
-
(USD MILLION) 117
-
INDIA: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
FOR EQUIPMENT, BY TYPE 2020–2027 (USD MILLION) 117
-
INDIA: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION)
-
118
-
INDIA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
INDIA: AUTOMATED CELL CULTURE EQUIPMENT
-
MARKET, BY END USER 2020–2027 (USD MILLION) 119
-
SOUTH KOREA:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027
-
(USD MILLION) 119
-
SOUTH KOREA: AUTOMATED CELL CULTURE EQUIPMENT MARKET,
-
BY PRODUCT TYPE 2020–2027 (USD MILLION) 119
-
SOUTH KOREA: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES, BY TYPE 2020–2027 (USD MILLION)
-
120
-
SOUTH KOREA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT,
-
BY TYPE 2020–2027 (USD MILLION) 120
-
SOUTH KOREA: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION)
-
121
-
SOUTH KOREA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
SOUTH KOREA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION) 122
-
REST
-
OF ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE,
-
(USD MILLION) 122
-
REST OF ASIA-PACIFIC: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 122
-
REST OF ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES,
-
BY TYPE 2020–2027
-
(USD MILLION) 123
-
REST OF ASIA-PACIFIC:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 123
-
REST OF ASIA-PACIFIC: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027 (USD MILLION) 124
-
TABLE
-
REST OF ASIA-PACIFIC: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY APPLICATION
-
REST OF ASIA-PACIFIC: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION) 125
-
MIDDLE EAST & AFRICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY
-
REGION, 2020–2027 (USD MILLION) 126
-
MIDDLE EAST & AFRICA:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB AUTOMATION TYPE, 2020–2027
-
(USD MILLION) 127
-
MIDDLE EAST & AFRICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 127
-
TABLE 180
-
MIDDLE EAST & AFRICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES,
-
BY TYPE 2020–2027
-
(USD MILLION) 127
-
MIDDLE EAST &
-
AFRICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027
-
(USD MILLION) 128
-
MIDDLE EAST & AFRICA: AUTOMATED CELL CULTURE
-
EQUIPMENT MARKET, FOR SOFTWARE, BY TYPE 2020–2027
-
(USD MILLION) 128
-
MIDDLE EAST & AFRICA: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY
-
APPLICATION 2020–2027 (USD MILLION) 129
-
MIDDLE EAST & AFRICA:
-
AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY END USER 2020–2027 (USD MILLION)
-
129
-
MIDDLE EAST: AUTOMATED CELL CULTURE EQUIPMENT MARKET, BY LAB
-
AUTOMATION TYPE, 2020–2027 (USD MILLION) 130
-
MIDDLE EAST: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, BY PRODUCT TYPE 2020–2027 (USD MILLION) 130
-
MIDDLE EAST: AUTOMATED CELL CULTURE EQUIPMENT MARKET, FOR CONSUMABLES,
-
BY TYPE 2020–2027 (USD MILLION) 130
-
MIDDLE EAST: AUTOMATED
-
CELL CULTURE EQUIPMENT MARKET, FOR EQUIPMENT, BY TYPE 2020–2027 (U








Leave a Comment