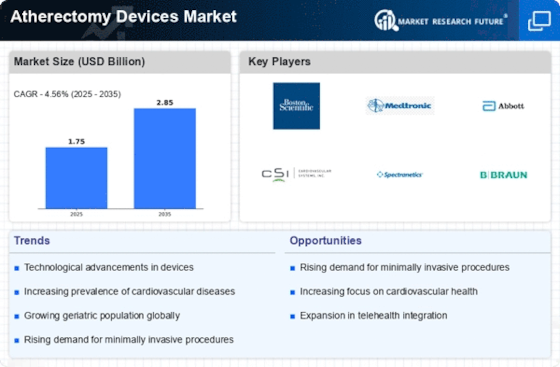
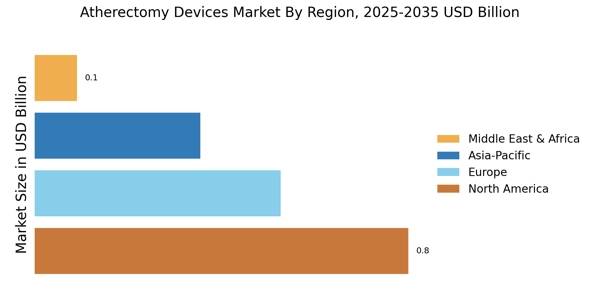
Rising Geriatric Population
The growing geriatric population is a significant factor influencing the Atherectomy Devices Market. As individuals age, they become more susceptible to cardiovascular diseases, including atherosclerosis, which often necessitates the use of atherectomy devices for treatment. The World Health Organization projects that the number of people aged 60 years and older will reach 2 billion by 2050, highlighting the increasing demand for effective medical interventions. This demographic shift is likely to drive the market for atherectomy devices, as healthcare systems adapt to the needs of an aging population, thereby creating opportunities for growth in this sector.
Technological Innovations in Atherectomy Devices
The Atherectomy Devices Market is experiencing a surge in technological innovations that enhance the efficacy and safety of procedures. Advanced imaging techniques, such as intravascular ultrasound and optical coherence tomography, are being integrated into atherectomy devices, allowing for real-time visualization of arterial blockages. This integration not only improves procedural outcomes but also reduces the risk of complications. Furthermore, the development of next-generation devices, which are designed to be more user-friendly and efficient, is likely to attract more healthcare providers. As a result, the market is projected to grow at a compound annual growth rate of approximately 8% over the next few years, driven by these technological advancements.
Increasing Incidence of Peripheral Artery Disease
The rising incidence of peripheral artery disease (PAD) is a critical driver for the Atherectomy Devices Market. As the population ages, the prevalence of PAD, which affects millions worldwide, continues to escalate. According to recent estimates, PAD affects around 8 to 12 million individuals in the United States alone. This growing patient population necessitates effective treatment options, including atherectomy procedures, which are known for their ability to restore blood flow in obstructed arteries. The increasing awareness of PAD and its associated risks is likely to propel the demand for atherectomy devices, thereby contributing to market growth.
Shift Towards Minimally Invasive Surgical Techniques
The Atherectomy Devices Market is witnessing a notable shift towards minimally invasive surgical techniques. Patients and healthcare providers alike are increasingly favoring procedures that minimize recovery time and reduce hospital stays. Atherectomy, being a less invasive option compared to traditional surgical methods, aligns well with this trend. The market is expected to benefit from this shift, as atherectomy devices offer effective solutions for treating vascular diseases with reduced trauma to the body. This trend is further supported by advancements in device design, which enhance the precision and effectiveness of minimally invasive procedures, potentially leading to a market growth rate of around 7% in the coming years.
Government Initiatives and Funding for Cardiovascular Health
Government initiatives aimed at improving cardiovascular health are playing a pivotal role in the Atherectomy Devices Market. Various health organizations and governments are investing in programs that promote awareness, prevention, and treatment of cardiovascular diseases. These initiatives often include funding for research and development of innovative medical devices, including atherectomy tools. Such support not only enhances the availability of advanced devices but also encourages healthcare providers to adopt these technologies. As a result, the market is likely to see increased investment and growth, driven by these supportive governmental policies and funding opportunities.