Market Trends
Key Emerging Trends in the Asia Pacific Cancer Immunotherapy Market
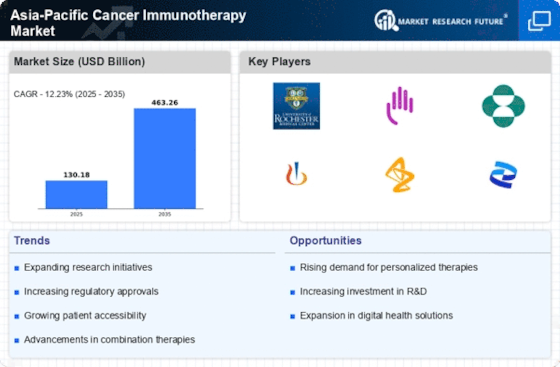
The Asia-Pacific cancer treatment sector is increasing rapidly as more individuals acquire cancer. Cases have increased as individuals live longer, make lifestyle changes, and understand more about cancer. This makes novel immunotherapies even more crucial.
In the Asia-Pacific, the market for genetic cancer immunotherapy is growing. Immune checkpoint inhibitors, monoclonal antibodies, and cell-based medicines are being used by more and more people because they work better for each patient. In the Asia-Pacific region, there are a number of government programs that help pay for the prices of cancer immunotherapy. Immunotherapeutics are getting more and more money to be studied, developed, and used. Because of this, the market grows, and more people can get better medical care.
The majority of cancer study is done in countries in Asia and the Pacific continent. More studies are being done right now to make sure that the immunotherapeutic drugs that were just found work and are safe. This both shows that they are committed to finding new ways to do things in the pharmaceutical industry and gives people access to cutting-edge drugs.
Even though they aren't very expensive, immune checkpoint inhibitors are becoming more and more famous in the Asia-Pacific region. The efficiency of standard cancer immunotherapies is increased when this suppressor is added to them. This category includes melanoma, lung, and bladder cancer. They boost immunity, which helps fight cancer. CAR-T therapy is an innovative new cancer treatment in Asia-Pacific gaining prevalence not only in the region but also worldwide. Cells are used in medicine. More individuals with some blood cancers and solid tumors are receiving this innovative therapy. They are considered major cancer therapeutic advances.
Cancer biosimilar manufacturing and approval have increased rapidly in this field. Biologics, which are very similar to approved biotech drugs, are cheaper. Thus, immunotherapeutic medicines will reach more people. Most novel cancer immunotherapy medicines in Asia-Pacific are patient-specific. Immunotherapeutic techniques that address patient quality of life, tailor treatments to individual patients, and change treatments depending on patient profiles are becoming increasingly important. Medical, educational, and pharmaceutical professionals are working together more. Collaboration simplifies resource, information, and tool sharing. This simplifies collaboration and accelerates immunotherapy development and sales. The economy affects the market, which affects cancer immunotherapies' advancement and availability. To help more people afford innovative medications, market players are changing how they set prices.
Despite progress, not everyone in Asia-Pacific has access to cancer care. Cities have more buildings and resources, improving accessibility. National difficulties may exist in various places. However, market participants appreciate eliminating these disparities.
COVID-19 has proved how important high-quality medical goods and services are. The Asian and Pacific cancer immunotherapy industry adjusted to the pandemic by focusing on online and home care and finding ways to ensure patients could still get immunotherapeutic treatments.

















Leave a Comment