Top Industry Leaders in the Asia Pacific Cancer Immunotherapy Market

BeiGene Receives First Approval for Anti-PD-1 Drug in APAC The company's drug, tislelizumab, has been approved in China for the treatment of locally advanced or metastatic urothelial carcinoma. This marks a significant step forward for BeiGene and paves the way for broader access to innovative immunotherapy options in the region.
AstraZeneca Expands Portfolio with Lynparza Approval in India PARP inhibitor is now approved in India for the maintenance treatment of adult patients with BRCA-mutated advanced ovarian cancer who have responded to complete platinum-based chemotherapy. This expands access to a crucial therapy for patients in India
Innovent Biologics Partners with Merck on PD-1 Inhibitor a leading Chinese biopharmaceutical company, has partnered with Merck to develop and commercialize KN032, a next-generation PD-1 inhibitor. This collaboration combines expertise and resources to accelerate the development of promising new therapies.
Jiangsu Hengrui Medicine and Shanghai Junshi Biosciences are developing and commercializing their own immunotherapy drugs, offering more affordable options and reducing reliance on imported medications.
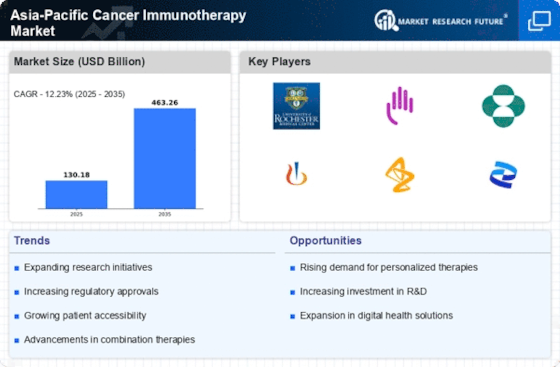
List of Asia Pacific Cancer Immunotherapy Key Companies in the Market
-
AmgenInc. (US)
-
Bristol-Myers Squibb Company (US)
-
Eli Lilly and Company (US)
-
AstraZeneca Plc. (UK)
-
GlaxoSmithKline Plc. (UK)
-
Bayer AG (Germany)
-
F. Hoffmann-La Roche Ltd. (Switzerland)
-
PfizerInc. (US)