Market Trends
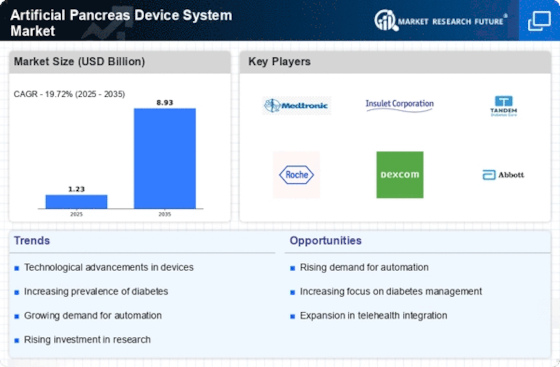
Key Emerging Trends in the Artificial Pancreas Device System Market
The artificial pancreas device system (APDS) industry is going through a transition that is showing a definite trend towards the control of diabetes. Another important development is the growing preference for intergrating CGM systems with insulin delivery systems in APDS or automated personalized diabetes systems. It enables the instant diagnosis of blood glucose and an adjustment of the insulin pump at the same time. The affected people are given an extremely automated method of glycemic control. This tendency fits to the common pattern of the personalized technology-driven diabetes management, where people are equipped with a more polished and automated-adjustable tool to impove blood glucose levels and relieve from the device of feeling stress and make processes slower.
In addition to the above, the second important aspect is the creation of closed-loop system that will be powered with hybrids. These systems offer the benefits that come with a combination of open-loop and closed-loop systems; they give users the best of both worlds by providing both flexibility and control over their insulin dosage while simultaneously taking advantage of the automation feature to maintain the glucose level within the optimal range. This is a sign of a strategic approach to diabetes management that takes into account the involvement of users with personalization, in addition to the benefits of automation.
Consequently, the APDS customer base has also got benefited tremendously and it is experiencing upgrades in algorithms. The move of APDS algorithms to be smarter is accelerating now and these means deploying machine learning and Artificial Intelligence to analyze complicated data profiles and then predict individualized insulin doses. Besides, such tendency to adjust and react to the ongoing variations of glucose metabolism and user demands strengthens the level of the efficiency of APDS at its operation.
Moreover, there has been a growing trend to build interoperability into the system which is crucial for the function of diabetes management solutions along with healthcare systems as one unit. Implementation leads to data sharing between devices and therefore forms a collaborative and widespread basis for diabetes care. This tendency coincides with the call for seamless health networks and patient-centered management system, which facilitate communication between the teams and services involved with diabetes care.
The APDS lends new discoveries here too, as the targeting toward pediatrician populations begins. Through the course of time and years as these systems are established and their safety and efficacy is accepted, the importance of APDS in the treatment of diabetes in children and adolescents is clearly seen. This testifies to the determination to target particular problems and use of modern tools that gives children and relatives the ability to handle the glycemic control in children well.

















Leave a Comment