Market Analysis
In-depth Analysis of Artificial Pancreas Device System Market Industry Landscape
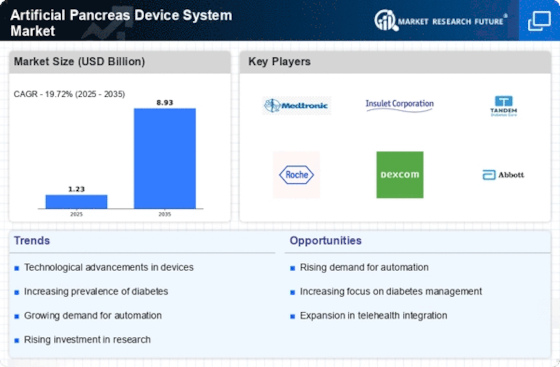
Artificial pancreas device system market is undergoing steady dynamics due to advances in technology, growing diabetes prevalence, and intense focus on the method of administering insulin. Technological innovation is a principal force that determines the patterns of competition in the pancreas device system market. These systems, also called as closed-loop systems, attempt to automate and perfect insulin delivery responding to real-time glucose levels that offer giving individuals with diabetes an enhanced control over their blood sugar levels. The presence of a CGM along with an insulin pump in the basic design of artificial pancreas system provides added precision and a better control of the glucose level.
Rising cases of diabetes, especially type 1 diabetes, is the key factor behind the development of markets for artificial pancreas device systems. The global increase in diabetes rates leads to a direct need for innovative technologies, which can be used to improve the process of insulin administration. Artificial pancreas systems have the advantage of a more systematic and automated administering of insulin. with the result that individuals with diabetes can be relieved of the burden of self-managing their diabetes and also of having unchecked diabetes.
Regulation lends a hand greatly in influencing how the man-made pancreas device system market will work. Stringent regulations and standards warrant that these medical devices that offer brand-new medicine ways are safe and effective, and of good quality. Compliance with regulatory requirements becomes an obligatory factor for approval of the products in the market, for increasing confidence among health professionals and patients, and to ensure the acceptance of the artificial pancreas system among general population.
The artificial pancreas devices system market dynamics are also driven by the shift from conventional diabetes care to patient-oriented and personalized treatments. As part of the bigger picture of diabetes self-care, the user-centered interface, connectivity features and data sharing function of these systems are key enablers in empowering people with diabetes to actively manage their condition. Artificial pancreas systems provide clarity of what's going on with glucose levels of an individual, making it possible to react timely and adjust adequately to prevent hypoglycemia and hyperglycemia.
The healthcare market dynamics also evolve in accordance with the economic conditions or reimbursement policies. The consideration of low cost and availability of artificial pancreas systems makes many people with diabetes and healthcare providers to accept them. Compendious repayment policies have consequences on product acceptance, use and market behavior at the global level. It is imperative for the economic sustainability of artificial pancreas programming for them to get the widespread adoption and the availability.

















Leave a Comment