Market Trends
Key Emerging Trends in the Alagille Syndrome Market
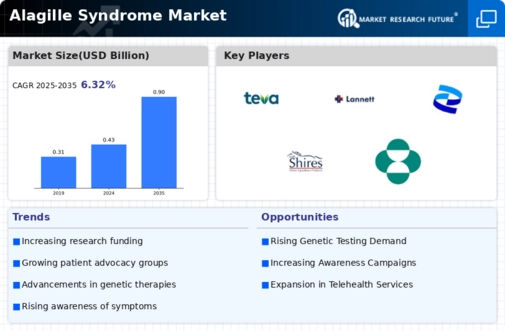
The demand for solutions in the US Alagille Syndrome market has witnessed a notable increase, reflecting a growing awareness and understanding of this rare genetic disorder. Alagille Syndrome is a rare genetic condition characterized by liver, heart, and other organ abnormalities, caused by mutations in the JAG1 or NOTCH2 genes. The rising demand can be attributed to various factors, including improved diagnostic capabilities, advancements in therapeutic options, and a concerted effort to address the specific needs of individuals affected by Alagille Syndrome.
Advancements in diagnostic capabilities have played a crucial role in meeting the demand for early and accurate identification of Alagille Syndrome. Genetic testing, including molecular testing for JAG1 and NOTCH2 gene mutations, has become a key tool in diagnosing this rare disorder. The ability to precisely identify the underlying genetic cause of Alagille Syndrome enables healthcare professionals to provide tailored management plans and support, contributing to the overall demand for solutions in the market.
The growing awareness of Alagille Syndrome among healthcare professionals, patients, and their families has also driven demand in the US market. As medical knowledge expands and information about rare genetic disorders becomes more accessible, there is an increasing recognition of the specific challenges posed by Alagille Syndrome. This heightened awareness has led to earlier and more accurate diagnoses, creating a greater demand for therapeutic interventions and supportive care to address the complex and varied symptoms associated with the syndrome.
The demand for solutions in the Alagille Syndrome market is further fueled by the development of therapeutic options aimed at managing the various manifestations of the disorder. While there is currently no cure for Alagille Syndrome, treatment approaches focus on alleviating specific symptoms and improving overall quality of life. Therapies targeting liver, heart, and other affected organs are being explored, and the market is witnessing ongoing research and clinical trials to expand the range of available treatment options.
Moreover, the emphasis on multidisciplinary care has become a central aspect of meeting the demand in the Alagille Syndrome market. Given the multi-organ nature of the disorder, collaboration among healthcare professionals from various specialties, including hepatologists, cardiologists, and genetic counselors, is essential. This comprehensive and coordinated approach ensures that individuals with Alagille Syndrome receive holistic care that addresses the diverse medical and developmental challenges associated with the disorder.
The supportive community and advocacy efforts for Alagille Syndrome have played a significant role in raising awareness, fostering research, and addressing the unique needs of individuals and families affected by the disorder. Support organizations provide valuable resources, connect affected individuals with expert medical care, and contribute to ongoing research initiatives. This collective effort has not only increased visibility but has also driven demand for improved diagnostic tools, therapeutic options, and supportive services within the Alagille Syndrome market.
The COVID-19 pandemic has highlighted the importance of telemedicine and remote healthcare services, especially for individuals with rare disorders like Alagille Syndrome. The adoption of virtual consultations, telehealth platforms, and remote monitoring tools has facilitated ongoing care and support for individuals with Alagille Syndrome, ensuring continuity of services during challenging times.


















Leave a Comment