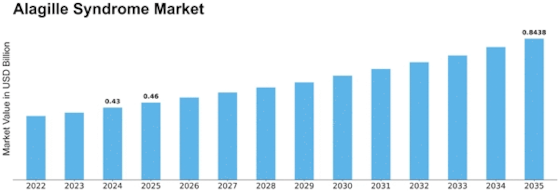
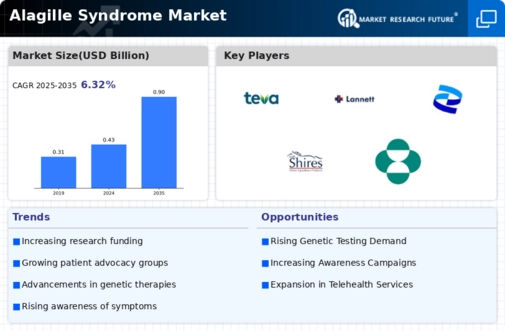
Alagille Syndrome Size
Alagille Syndrome Market Growth Projections and Opportunities
The Alagille Syndrome market, a niche within the healthcare landscape, witnesses a range of market share positioning strategies as companies navigate the challenges associated with this rare genetic disorder. A fundamental strategy in this specialized field is product differentiation, where pharmaceutical companies focus on developing innovative and targeted therapies for Alagille Syndrome. This may include medications designed to address specific symptoms, advanced gene therapies, or supportive care treatments. By offering unique and tailored solutions, companies aim to distinguish themselves in a competitive market, positioning their brand as a leader in providing effective and specialized interventions for Alagille Syndrome patients.
Cost leadership is another critical strategy in the Alagille Syndrome market, recognizing the importance of affordability and accessibility in rare disease treatments. Companies strive to optimize the costs associated with medications, diagnostic tools, and supportive care without compromising on the quality of interventions. This involves negotiating favorable agreements with suppliers, exploring cost-effective manufacturing processes, and implementing efficient distribution channels. By providing cost-efficient options, companies not only attract a broader patient base but also contribute to an increased market share by addressing the economic considerations of patients, healthcare providers, and payers.
Market segmentation plays a pivotal role in strategic positioning within the Alagille Syndrome market. Companies recognize the diversity of symptoms and patient needs associated with this disorder and tailor their interventions to address specific aspects of Alagille Syndrome. For instance, developing therapies targeting liver complications, cardiac issues, or addressing developmental challenges allows companies to effectively address niche markets within the broader Alagille Syndrome patient population. This targeted approach ensures that the interventions align with the specific requirements of different patients, enhancing the overall market presence of these companies.
Strategic collaborations and partnerships are integral to the Alagille Syndrome market, given the rarity and complexity of the disorder. Companies often engage with research institutions, patient advocacy groups, and healthcare providers to enhance their understanding of Alagille Syndrome, access valuable patient data, and expedite the development of new treatments. Collaborative efforts not only contribute to scientific advancements but also position companies as leaders in the field, reinforcing their market share in the challenging landscape of rare disease therapeutics.
Customer-centric strategies are paramount in the Alagille Syndrome market, where patients and their families face unique challenges associated with a rare genetic disorder. Companies that prioritize patient education, provide comprehensive support programs, and ensure clear communication about treatment options contribute to increased patient satisfaction and loyalty. Building strong relationships with healthcare providers and patient advocacy groups through awareness campaigns and continuous improvement of patient support services positively influences the market share of companies offering interventions for Alagille Syndrome.
Geographic expansion is a crucial component of market share positioning in the Alagille Syndrome market. Companies often target regions with a higher prevalence of Alagille Syndrome cases or where there is a growing demand for specialized treatments. Establishing a robust presence in key markets allows companies to adapt their interventions to regional variations in healthcare practices and preferences, contributing to an increased market share on a global scale.


















Leave a Comment