Market Growth Projections
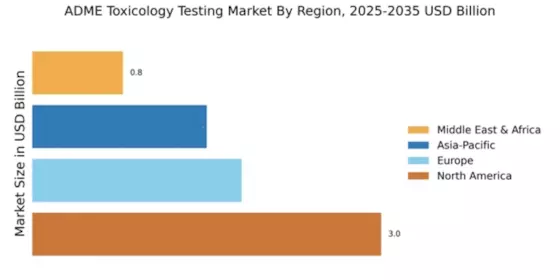
The Global ADME Toxicology Testing Market Industry is projected to experience substantial growth over the coming years. The market is expected to expand from 0.48 USD Billion in 2024 to an impressive 4.25 USD Billion by 2035, reflecting a compound annual growth rate (CAGR) of 21.96% from 2025 to 2035. This growth trajectory is indicative of the increasing importance of ADME testing in drug development and regulatory compliance. As the pharmaceutical landscape evolves, the demand for reliable toxicology testing solutions is likely to intensify, further driving market expansion. Stakeholders are advised to remain vigilant and responsive to these trends to capitalize on emerging opportunities.
Rising Demand for Drug Development
The Global ADME Toxicology Testing Market Industry experiences heightened demand due to the increasing need for efficient drug development processes. Pharmaceutical companies are under pressure to expedite the development of new therapeutics while ensuring safety and efficacy. This demand is reflected in the projected market growth from 0.48 USD Billion in 2024 to an anticipated 4.25 USD Billion by 2035, indicating a robust compound annual growth rate (CAGR) of 21.96% from 2025 to 2035. As regulatory agencies emphasize the importance of ADME testing in drug approval processes, the industry is poised for substantial expansion.
Emerging Markets and Global Expansion
Emerging markets are becoming increasingly important in the Global ADME Toxicology Testing Market Industry. Countries in Asia-Pacific and Latin America are witnessing a surge in pharmaceutical research and development activities, driven by growing investments in healthcare infrastructure and increasing access to advanced technologies. This trend is expected to contribute to the overall market growth, as companies seek to establish a presence in these regions. The expansion into emerging markets not only provides access to new customer bases but also offers opportunities for collaboration with local research institutions. As these markets continue to evolve, they are likely to play a crucial role in shaping the future of ADME toxicology testing.
Increasing Focus on Personalized Medicine
The shift towards personalized medicine is reshaping the Global ADME Toxicology Testing Market Industry. As healthcare moves towards tailored therapies, understanding individual variations in drug metabolism and toxicity becomes crucial. ADME testing provides essential insights into how different populations respond to medications, enabling the development of safer and more effective treatments. This trend is particularly relevant as the market is projected to grow significantly, with estimates suggesting a rise from 0.48 USD Billion in 2024 to 4.25 USD Billion by 2035. The increasing emphasis on personalized approaches in drug development is likely to drive demand for advanced ADME testing solutions.
Regulatory Compliance and Safety Standards
The Global ADME Toxicology Testing Market Industry is significantly influenced by stringent regulatory compliance and safety standards imposed by health authorities worldwide. Regulatory agencies, such as the FDA and EMA, require comprehensive toxicological data to ensure the safety of new drugs before they reach the market. This necessity drives pharmaceutical companies to invest in ADME testing to meet these regulatory demands. As the landscape of drug approval becomes increasingly complex, the emphasis on thorough toxicological evaluations is expected to bolster the market. Companies that prioritize compliance are likely to gain a competitive edge, further propelling the industry's growth.
Technological Advancements in Testing Methods
Technological innovations play a pivotal role in shaping the Global ADME Toxicology Testing Market Industry. The integration of high-throughput screening, in silico modeling, and advanced bioanalytical techniques enhances the accuracy and efficiency of toxicology assessments. These advancements not only reduce the time required for testing but also improve the predictive capabilities of ADME studies. As a result, pharmaceutical companies are increasingly adopting these technologies to streamline their drug development pipelines. The ongoing evolution of testing methodologies is likely to contribute significantly to market growth, as stakeholders seek to leverage cutting-edge solutions for regulatory compliance and risk assessment.