Emerging Regulatory Frameworks
The evolving regulatory landscape is shaping the adme toxicology-testing market. Regulatory agencies in the US are increasingly emphasizing the importance of thorough toxicology assessments in the drug approval process. New guidelines and frameworks are being established to ensure that safety evaluations are comprehensive and scientifically sound. This shift is likely to increase the demand for adme toxicology-testing services, as companies must comply with stringent regulations to bring their products to market. The emphasis on data integrity and transparency in testing results is expected to further drive the growth of the adme toxicology-testing market, as stakeholders seek to meet regulatory expectations.
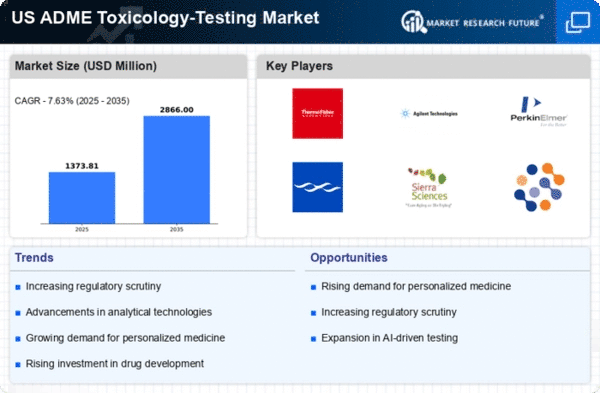
Rising Demand for Drug Development
The ADME Toxicology-Testing Market is experiencing a surge in demand driven by the increasing need for drug development. Pharmaceutical companies are under pressure to expedite the drug discovery process while ensuring safety and efficacy. In 2025, the market for drug development is projected to reach approximately $200 billion in the US, necessitating robust toxicology testing to mitigate risks associated with new compounds. This trend is likely to propel the adme toxicology-testing market as companies seek reliable testing methods to comply with regulatory requirements and to enhance their product pipelines. The emphasis on early-stage testing to identify potential toxic effects is becoming a standard practice, thereby increasing the reliance on advanced toxicology testing solutions.
Growing Investment in Biopharmaceuticals
Investment in biopharmaceuticals is a key driver for the adme toxicology-testing market. The biopharmaceutical sector is rapidly expanding, with the US market projected to reach $400 billion by 2025. This growth is accompanied by an increasing need for comprehensive toxicology testing to evaluate the safety of biologics and biosimilars. As companies invest innovative therapies, the demand for reliable adme toxicology-testing solutions is likely to rise. The complexity of biopharmaceuticals necessitates advanced testing methodologies to assess their pharmacokinetics and potential toxic effects, thereby creating opportunities for growth within the adme toxicology-testing market.
Increased Focus on Personalized Medicine
The shift towards personalized medicine is significantly influencing the adme toxicology-testing market. As healthcare moves towards tailored therapies, understanding individual responses to drugs becomes crucial. This trend is expected to drive the demand for sophisticated toxicology testing methods that can assess the pharmacokinetics and toxicity of drugs in diverse populations. By 2025, the personalized medicine market is anticipated to exceed $100 billion in the US, which will likely necessitate enhanced toxicology testing to ensure patient safety. The ADME Toxicology-Testing Market is poised to benefit from this focus, as it provides essential data that informs drug development and patient-specific treatment plans.
Technological Innovations in Testing Methods
Technological advancements are revolutionizing the adme toxicology-testing market. Innovations such as high-throughput screening, in vitro testing, and computational modeling are enhancing the efficiency and accuracy of toxicology assessments. These technologies enable faster and more reliable evaluations of drug safety, which is crucial in the competitive pharmaceutical landscape. As the adme toxicology-testing market adapts to these innovations, it is likely to see increased adoption of automated systems and data analytics tools. By 2025, the integration of these technologies is expected to streamline testing processes, reduce costs, and improve the overall quality of toxicology data, thereby benefiting stakeholders across the industry.