Advancements in Drug Development
Innovations in drug development and biotechnology are significantly influencing the Adalimumab Drug Market. The ongoing research and development efforts aimed at enhancing the efficacy and safety profiles of existing therapies are likely to yield new formulations and delivery methods. These advancements may include improved dosing regimens and combination therapies that could enhance patient outcomes. The Adalimumab Drug Market stands to gain from these developments, as pharmaceutical companies invest in cutting-edge technologies to optimize treatment options. Additionally, the introduction of novel delivery systems, such as autoinjectors and prefilled syringes, may improve patient adherence and satisfaction. As a result, the Adalimumab Drug Market is poised for growth, driven by the continuous evolution of drug development practices.
Regulatory Support for Biologics
Regulatory agencies are increasingly supportive of biologic therapies, which is a significant driver for the Adalimumab Drug Market. The streamlined approval processes and favorable policies for biologics are likely to facilitate the introduction of new products and indications. This regulatory environment encourages pharmaceutical companies to invest in the development of innovative therapies, including those based on Adalimumab. Moreover, the growing acceptance of biosimilars is expected to enhance competition within the Adalimumab Drug Market, potentially leading to lower prices and increased accessibility for patients. As regulatory frameworks continue to evolve, the Adalimumab Drug Market is well-positioned to capitalize on these opportunities, fostering growth and innovation in the sector.
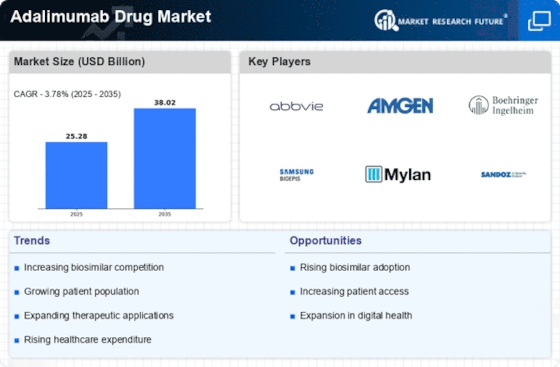
Increasing Healthcare Expenditure
The rise in healthcare expenditure across various regions is a crucial factor propelling the Adalimumab Drug Market. As governments and private sectors allocate more resources to healthcare, there is a corresponding increase in the availability of advanced treatment options for patients. This trend is particularly evident in developed economies, where healthcare budgets are expanding to accommodate innovative therapies. The Adalimumab Drug Market benefits from this increased spending, as more patients gain access to biologic treatments that were previously unaffordable. Furthermore, the emphasis on value-based care is likely to drive investments in effective therapies, including Adalimumab, which has demonstrated substantial clinical benefits. Consequently, the Adalimumab Drug Market is expected to thrive in an environment characterized by rising healthcare investments.
Patient Demand for Effective Treatments
The increasing patient demand for effective and convenient treatment options is a driving force in the Adalimumab Drug Market. Patients are becoming more informed about their health conditions and are actively seeking therapies that offer better outcomes and improved quality of life. This trend is particularly evident in chronic conditions, where patients often require long-term management strategies. The Adalimumab Drug Market is responding to this demand by providing therapies that not only address symptoms but also target the underlying causes of diseases. Additionally, the focus on personalized medicine is likely to enhance patient engagement and adherence to treatment regimens. As patients continue to advocate for effective therapies, the Adalimumab Drug Market is expected to grow in response to this evolving landscape.
Rising Prevalence of Autoimmune Diseases
The increasing incidence of autoimmune diseases such as rheumatoid arthritis, psoriasis, and Crohn's disease is a primary driver for the Adalimumab Drug Market. According to recent data, autoimmune diseases affect millions of individuals worldwide, leading to a heightened demand for effective treatment options. This trend is likely to continue, as the prevalence of these conditions appears to be on the rise. The Adalimumab Drug Market is positioned to benefit from this growing patient population, as healthcare providers seek reliable therapies to manage these chronic conditions. Furthermore, the increasing awareness and diagnosis of autoimmune diseases contribute to the market's expansion, as more patients are being identified and treated. As a result, the Adalimumab Drug Market is expected to experience sustained growth in response to this rising demand.