Market Growth Projections
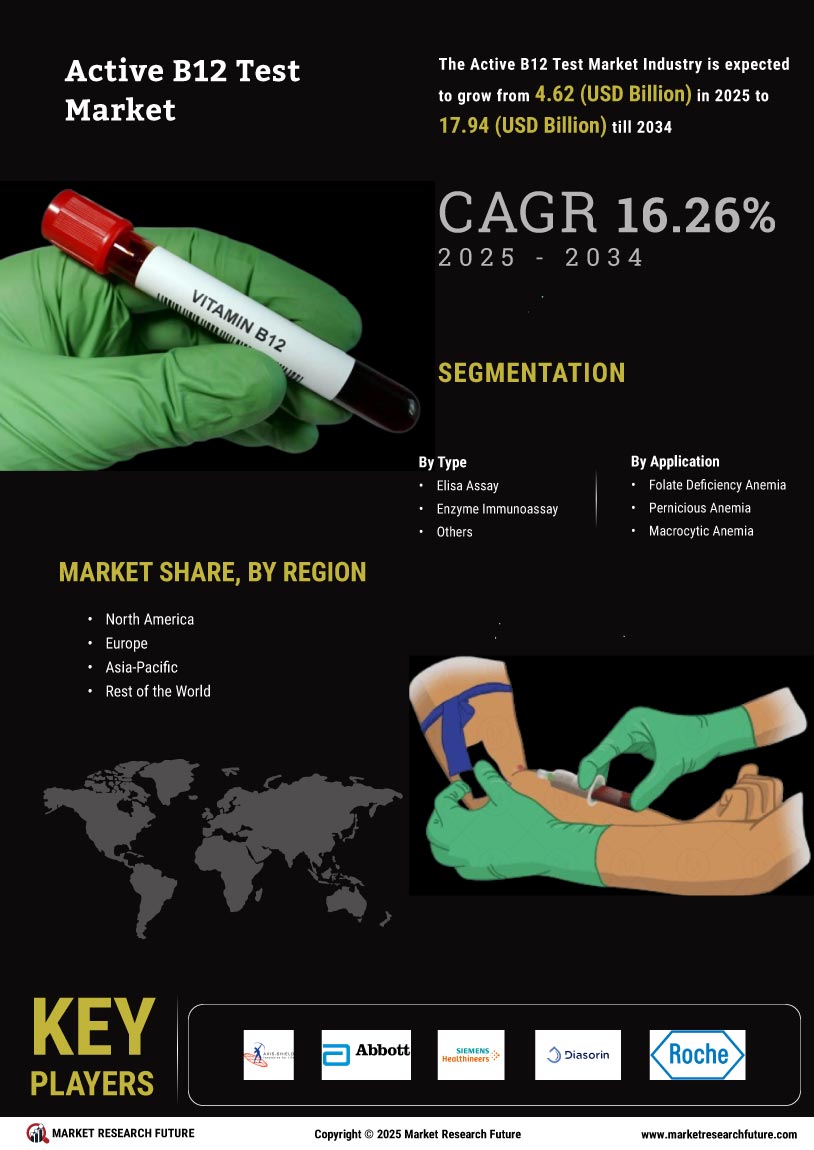
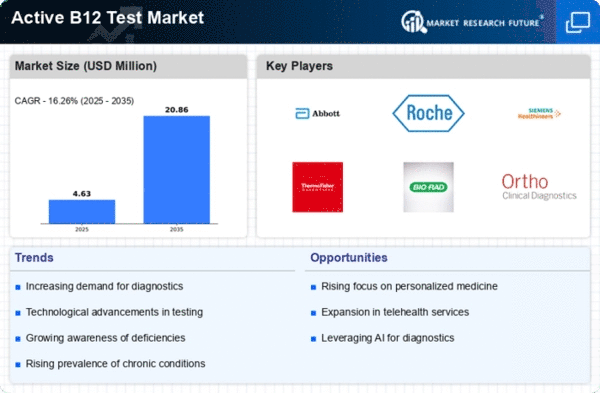
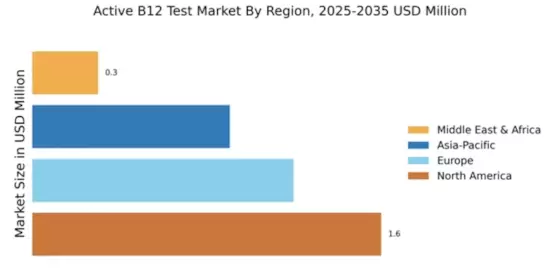
The Global Active B12 Test Market Industry is projected to experience substantial growth over the next decade. With a market value of 3.98 USD Billion in 2024, it is expected to reach 20.9 USD Billion by 2035, reflecting a compound annual growth rate of 16.25% from 2025 to 2035. This growth trajectory indicates a robust demand for active B12 testing, driven by factors such as increased awareness of vitamin deficiencies, advancements in diagnostic technologies, and supportive regulatory frameworks. The market's expansion is indicative of a broader trend towards preventive healthcare and improved nutritional awareness.
Regulatory Support for Nutritional Testing
Regulatory bodies are increasingly recognizing the importance of nutritional testing, which is positively influencing the Global Active B12 Test Market Industry. Policies that promote routine screening for vitamin deficiencies are being implemented in various countries. This regulatory support encourages healthcare providers to incorporate active B12 testing into standard practice. As a result, the market is likely to experience growth as more individuals gain access to testing services. The alignment of healthcare policies with nutritional health initiatives suggests a promising future for the active B12 testing sector.
Rising Prevalence of Vitamin B12 Deficiency
The increasing incidence of vitamin B12 deficiency globally is a primary driver for the Global Active B12 Test Market Industry. Factors such as dietary changes, particularly in vegetarian and vegan populations, contribute to this trend. According to health organizations, deficiencies can lead to severe neurological and cognitive issues. As awareness of these health risks grows, healthcare providers are more likely to recommend active B12 testing. This heightened demand is expected to propel the market, with projections indicating a market value of 3.98 USD Billion in 2024, reflecting the urgent need for effective diagnostic solutions.
Aging Population and Associated Health Risks
The aging global population is a crucial factor impacting the Global Active B12 Test Market Industry. Older adults are at a higher risk for vitamin B12 deficiency due to factors such as decreased absorption and dietary limitations. As the demographic of individuals aged 65 and older continues to grow, there is an increasing need for effective screening and diagnostic tools. This trend is likely to drive demand for active B12 testing, as healthcare systems adapt to the needs of an aging population. Consequently, the market is expected to witness substantial growth in the coming years.
Technological Advancements in Diagnostic Testing
Innovations in diagnostic technologies are significantly influencing the Global Active B12 Test Market Industry. The introduction of more accurate and efficient testing methods, such as high-performance liquid chromatography and mass spectrometry, enhances the reliability of B12 assessments. These advancements not only improve patient outcomes but also streamline laboratory processes, making testing more accessible. As healthcare facilities adopt these technologies, the market is poised for growth, with an anticipated compound annual growth rate of 16.25% from 2025 to 2035. This trend underscores the importance of integrating cutting-edge technology in routine diagnostics.
Increased Awareness and Education on Nutritional Health
There is a growing emphasis on nutritional health and preventive care, which is driving the Global Active B12 Test Market Industry. Public health campaigns and educational initiatives aimed at informing populations about the importance of vitamin B12 are becoming more prevalent. This increased awareness leads to higher testing rates as individuals seek to understand their nutritional status. Healthcare providers are also more proactive in recommending testing as part of routine health assessments. As a result, the market is expected to expand significantly, with projections indicating a value of 20.9 USD Billion by 2035.