Growing Geriatric Population
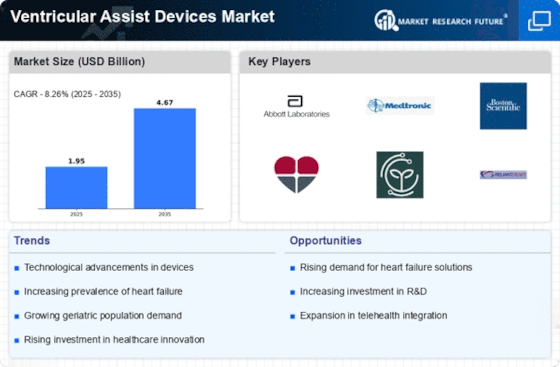
The increasing geriatric population is a significant driver for the Ventricular Assist Devices Market. As individuals age, the risk of developing heart-related conditions, including heart failure, escalates. This demographic shift is expected to lead to a higher demand for ventricular assist devices as older adults often require advanced medical interventions to manage their health. According to demographic studies, the proportion of individuals aged 65 and older is projected to rise substantially in the coming years. This trend suggests that healthcare systems will need to adapt to accommodate the needs of an aging population, thereby creating opportunities for growth within the Ventricular Assist Devices Market.
Rising Healthcare Expenditure
Rising healthcare expenditure is another critical factor influencing the Ventricular Assist Devices Market. As countries allocate more resources to healthcare, there is a growing emphasis on advanced medical technologies that can improve patient outcomes. Increased funding for cardiovascular care, including the development and implementation of ventricular assist devices, is likely to enhance the availability and accessibility of these life-saving technologies. Moreover, as healthcare systems recognize the long-term cost savings associated with effective heart failure management, investments in ventricular assist devices may become a priority. This trend indicates a positive outlook for the Ventricular Assist Devices Market, as financial support for innovative solutions continues to grow.
Supportive Regulatory Frameworks
Supportive regulatory frameworks play a crucial role in the expansion of the Ventricular Assist Devices Market. Regulatory bodies are increasingly recognizing the importance of these devices in managing advanced heart failure, leading to streamlined approval processes and enhanced market access. For example, expedited pathways for device approval can significantly reduce the time it takes for new technologies to reach the market. This regulatory support not only encourages manufacturers to invest in research and development but also fosters competition, which can lead to better products and lower costs for patients. As a result, the Ventricular Assist Devices Market is likely to benefit from a more favorable regulatory environment that promotes innovation and accessibility.
Increasing Prevalence of Heart Failure
The rising incidence of heart failure is a primary driver for the Ventricular Assist Devices Market. As heart failure cases continue to escalate, the demand for effective treatment options, including ventricular assist devices, is likely to increase. According to recent estimates, heart failure affects millions of individuals worldwide, leading to a significant burden on healthcare systems. This growing patient population necessitates innovative solutions to manage heart failure effectively. Consequently, the Ventricular Assist Devices Market is poised for growth as healthcare providers seek to implement advanced technologies to improve patient outcomes and quality of life. The increasing prevalence of heart failure may also stimulate research and development efforts, further enhancing the market landscape.
Technological Innovations in Device Design
Technological advancements in the design and functionality of ventricular assist devices are propelling the Ventricular Assist Devices Market forward. Innovations such as miniaturization, improved biocompatibility, and enhanced durability are making these devices more effective and user-friendly. For instance, the introduction of next-generation devices that offer better hemodynamic performance and reduced complications is likely to attract more patients and healthcare providers. Furthermore, the integration of remote monitoring technologies allows for real-time patient data analysis, which can lead to timely interventions. As these technological innovations continue to evolve, they are expected to expand the applications of ventricular assist devices, thereby driving growth in the market.