Growing Focus on Personalized Medicine
The Vasomotor Symptoms Market is increasingly shifting towards personalized medicine, which tailors treatment plans to individual patient needs. This approach is gaining traction as healthcare providers recognize the variability in how women experience vasomotor symptoms. Personalized treatment strategies may include a combination of lifestyle modifications, pharmacological interventions, and alternative therapies. Market data suggests that personalized medicine could enhance patient satisfaction and treatment adherence, potentially leading to better health outcomes. As the healthcare landscape evolves, the emphasis on personalized approaches is likely to drive innovation and growth within the Vasomotor Symptoms Market, as companies strive to meet the unique needs of their patients.
Advancements in Hormone Replacement Therapy
Recent advancements in hormone replacement therapy (HRT) are significantly influencing the Vasomotor Symptoms Market. New formulations and delivery methods have emerged, offering improved efficacy and reduced side effects. For instance, transdermal patches and bioidentical hormones are gaining traction among healthcare providers and patients alike. According to market data, the HRT segment is projected to witness substantial growth, with an estimated CAGR of 6% over the next five years. This growth is attributed to the increasing acceptance of HRT as a viable treatment option for managing vasomotor symptoms. As more women seek relief from these symptoms, the demand for advanced HRT solutions is expected to rise, further propelling the market.
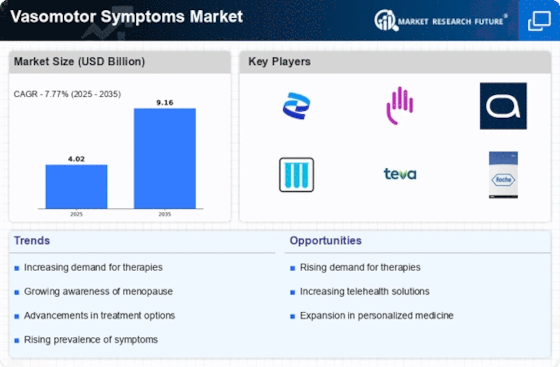
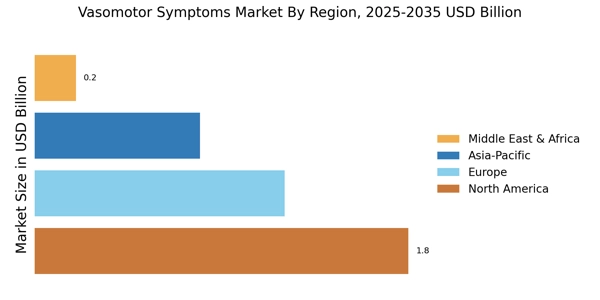
Increasing Prevalence of Vasomotor Symptoms
The Vasomotor Symptoms Market is experiencing a notable surge in demand due to the increasing prevalence of vasomotor symptoms, particularly among menopausal women. Research indicates that approximately 75% of women undergoing menopause report experiencing these symptoms, which include hot flashes and night sweats. This rising incidence is prompting healthcare providers to seek effective treatment options, thereby driving market growth. Furthermore, the aging population is contributing to this trend, as the number of women entering menopause continues to rise. As a result, pharmaceutical companies are focusing on developing innovative therapies to address these symptoms, which is likely to enhance the overall market landscape.
Increased Investment in Women's Health Research
The Vasomotor Symptoms Market is benefiting from increased investment in women's health research, which is gaining recognition as a critical area of focus. Governments and private organizations are allocating more resources to study the physiological and psychological aspects of vasomotor symptoms. This heightened interest is likely to lead to the development of novel therapies and treatment protocols. Recent funding initiatives have aimed at understanding the long-term effects of vasomotor symptoms and their impact on women's quality of life. As research progresses, it is anticipated that new insights will emerge, potentially transforming the treatment landscape and driving growth in the Vasomotor Symptoms Market.
Rising Demand for Non-Hormonal Treatment Options
There is a growing demand for non-hormonal treatment options within the Vasomotor Symptoms Market, driven by concerns over the risks associated with hormone therapy. Many women are seeking alternatives due to personal health considerations or contraindications for HRT. Non-hormonal therapies, such as selective serotonin reuptake inhibitors (SSRIs) and herbal supplements, are gaining popularity as effective options for managing vasomotor symptoms. Market analysis indicates that the non-hormonal segment is expected to grow at a CAGR of 5% over the next few years, reflecting the shift in patient preferences. This trend is prompting pharmaceutical companies to invest in research and development of new non-hormonal therapies, thereby expanding the market.