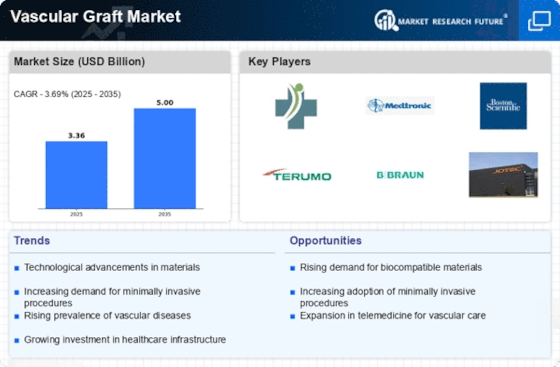
Top Industry Leaders in the Vascular Graft Market

Latest Vascular Graft Companies Updates:
Terumo: Received FDA approval for the Aortic Zenith™ TX2 device, an expanded indication of their existing Zenith™ platform, offering a minimally invasive option for repairing complex aortic arch aneurysms.
W. L. Gore & Associates: Introduced the Excluder™ AAA System with Tri-Seal Anchoring System, featuring enhanced anchoring technology for improved long-term outcomes in abdominal aortic aneurysm (AAA) repair.
Getinge: Developed the Maquet Biograft™ Hybrid AAA Stent-Graft, combining a synthetic stent-graft with a collagen-based endothelial lining, aiming to reduce the risk of complications associated with traditional grafts.
BD: Acquired C. R. Bard, Inc., gaining access to Bard's vascular access and peripheral intervention portfolio, further solidifying its position in the cardiovascular device market.Abbott: Partnered with Cardival Biosciences to develop and commercialize novel bioresorbable vascular scaffolds, offering a next-generation alternative to traditional stents that eventually need removal.
List of Vascular Graft Key companies in the market:
- Cardinal Health (US)
- Gore Medical (US),
- Terumo (Japan)
- C. R. Bard (US)
- Medtronic (Ireland)
- LeMaitre Vascular (US)
- B. Braun Melsugen AG (Germany)
- Endologix (US)
- Maquet (Germany)
- Cook Medical (US)