Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
Direct Sales
Online Sales
Third-party Distributors
North America
Europe
South America
Asia Pacific
Middle East and Africa
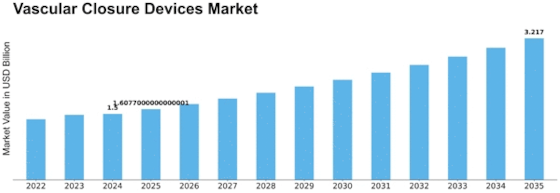
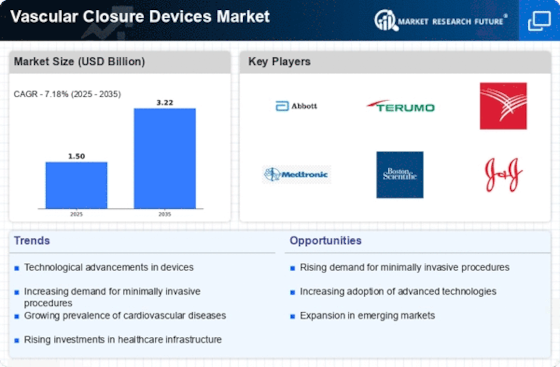
North America Outlook (USD Billion, 2019-2035)
North America Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
North America Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
North America Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
North America Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
North America Vascular Closure Devices Market by Regional Type
US
Canada
US Outlook (USD Billion, 2019-2035)
US Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
US Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
US Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
US Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
CANADA Outlook (USD Billion, 2019-2035)
CANADA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
CANADA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
CANADA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
CANADA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
Europe Outlook (USD Billion, 2019-2035)
Europe Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
Europe Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
Europe Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
Europe Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
Europe Vascular Closure Devices Market by Regional Type
Germany
UK
France
Russia
Italy
Spain
Rest of Europe
GERMANY Outlook (USD Billion, 2019-2035)
GERMANY Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
GERMANY Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
GERMANY Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
GERMANY Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
UK Outlook (USD Billion, 2019-2035)
UK Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
UK Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
UK Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
UK Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
FRANCE Outlook (USD Billion, 2019-2035)
FRANCE Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
FRANCE Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
FRANCE Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
FRANCE Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
RUSSIA Outlook (USD Billion, 2019-2035)
RUSSIA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
RUSSIA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
RUSSIA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
RUSSIA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
ITALY Outlook (USD Billion, 2019-2035)
ITALY Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
ITALY Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
ITALY Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
ITALY Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
SPAIN Outlook (USD Billion, 2019-2035)
SPAIN Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
SPAIN Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
SPAIN Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
SPAIN Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
REST OF EUROPE Outlook (USD Billion, 2019-2035)
REST OF EUROPE Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
REST OF EUROPE Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
REST OF EUROPE Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
REST OF EUROPE Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
APAC Outlook (USD Billion, 2019-2035)
APAC Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
APAC Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
APAC Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
APAC Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
APAC Vascular Closure Devices Market by Regional Type
China
India
Japan
South Korea
Malaysia
Thailand
Indonesia
Rest of APAC
CHINA Outlook (USD Billion, 2019-2035)
CHINA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
CHINA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
CHINA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
CHINA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
INDIA Outlook (USD Billion, 2019-2035)
INDIA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
INDIA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
INDIA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
INDIA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
JAPAN Outlook (USD Billion, 2019-2035)
JAPAN Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
JAPAN Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
JAPAN Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
JAPAN Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
SOUTH KOREA Outlook (USD Billion, 2019-2035)
SOUTH KOREA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
SOUTH KOREA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
SOUTH KOREA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
SOUTH KOREA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
MALAYSIA Outlook (USD Billion, 2019-2035)
MALAYSIA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
MALAYSIA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
MALAYSIA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
MALAYSIA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
THAILAND Outlook (USD Billion, 2019-2035)
THAILAND Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
THAILAND Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
THAILAND Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
THAILAND Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
INDONESIA Outlook (USD Billion, 2019-2035)
INDONESIA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
INDONESIA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
INDONESIA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
INDONESIA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
REST OF APAC Outlook (USD Billion, 2019-2035)
REST OF APAC Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
REST OF APAC Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
REST OF APAC Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
REST OF APAC Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
South America Outlook (USD Billion, 2019-2035)
South America Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
South America Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
South America Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
South America Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
South America Vascular Closure Devices Market by Regional Type
Brazil
Mexico
Argentina
Rest of South America
BRAZIL Outlook (USD Billion, 2019-2035)
BRAZIL Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
BRAZIL Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
BRAZIL Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
BRAZIL Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
MEXICO Outlook (USD Billion, 2019-2035)
MEXICO Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
MEXICO Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
MEXICO Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
MEXICO Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
ARGENTINA Outlook (USD Billion, 2019-2035)
ARGENTINA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
ARGENTINA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
ARGENTINA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
ARGENTINA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
REST OF SOUTH AMERICA Outlook (USD Billion, 2019-2035)
REST OF SOUTH AMERICA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
REST OF SOUTH AMERICA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
REST OF SOUTH AMERICA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
REST OF SOUTH AMERICA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
MEA Outlook (USD Billion, 2019-2035)
MEA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
MEA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
MEA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
MEA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
MEA Vascular Closure Devices Market by Regional Type
GCC Countries
South Africa
Rest of MEA
GCC COUNTRIES Outlook (USD Billion, 2019-2035)
GCC COUNTRIES Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
GCC COUNTRIES Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
GCC COUNTRIES Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
GCC COUNTRIES Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
SOUTH AFRICA Outlook (USD Billion, 2019-2035)
SOUTH AFRICA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
SOUTH AFRICA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
SOUTH AFRICA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
SOUTH AFRICA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors
REST OF MEA Outlook (USD Billion, 2019-2035)
REST OF MEA Vascular Closure Devices Market by Device Type
Passive Closure Devices
Active Closure Devices
Suture Closure Devices
Collagen-based Closure Devices
REST OF MEA Vascular Closure Devices Market by Procedure Type
Transvascular Procedures
Cardiac Procedures
Peripheral Procedures
REST OF MEA Vascular Closure Devices Market by End User Type
Hospitals
Ambulatory Surgical Centers
Specialty Clinics
REST OF MEA Vascular Closure Devices Market by Distribution Channel Type
Direct Sales
Online Sales
Third-party Distributors

















Leave a Comment