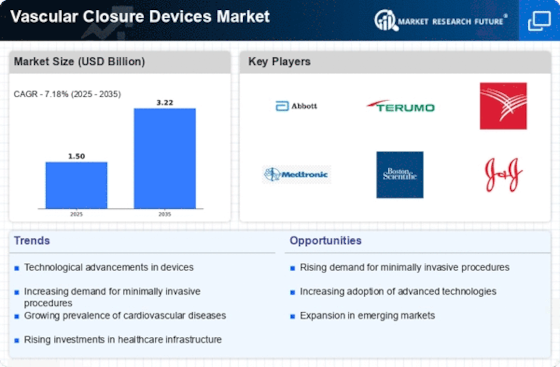
Top Industry Leaders in the Vascular Closure Devices Market

Latest Vascular Closure Devices Companies Updates:
Abbott announced positive clinical trial results for their Amplify™ Seal vascular closure device, demonstrating high closure rates and minimal complications. This could strengthen their position in the market.
Terumo Corporation launched their Tri-Clover™ device in Europe, offering a sutureless closure option for femoral artery access sites. This expands their portfolio and targets a growing segment.
Cardiva Medical, Inc. received FDA approval for their VASCADE™ Closure System, the first collagen-based closure device specifically designed for radial artery access. This opens up new opportunities in interventional cardiology procedures.
Cook Medical received CE Mark approval for their NaviSeal™ Advanced Closure System, featuring real-time ultrasound guidance for improved device placement and closure success. This marks a technological advancement in the market.
Tricol Biomedical and Vivasure Medical Ltd are focusing on developing next-generation closure devices with enhanced features and broader applicability.
List of Vascular Closure Devices Key companies in the market:
-
Terumo Corporation
-
Cardinal Health Inc.
-
Cardiva Medical Inc.
-
Morris Innovative Inc.
-
Medtronic plc
-
Essential Medical Inc.
-
Merit Medical Systems Inc.