Government Funding and Support
Government funding plays a pivotal role in the US Tuberculosis Vaccine Treatment Market. The federal government, through agencies such as the National Institutes of Health (NIH) and the CDC, allocates substantial resources to TB research and vaccine development. In recent years, funding for TB-related initiatives has increased, reflecting a commitment to combatting this public health challenge. For instance, the NIH has invested millions in grants aimed at fostering innovative vaccine research. This financial support not only encourages the development of new vaccines but also facilitates clinical trials and regulatory approvals. As a result, the availability of government funding is a significant driver for the growth of the US Tuberculosis Vaccine Treatment Market, enabling researchers and companies to advance their projects and bring effective vaccines to market.
Rising Incidence of Tuberculosis
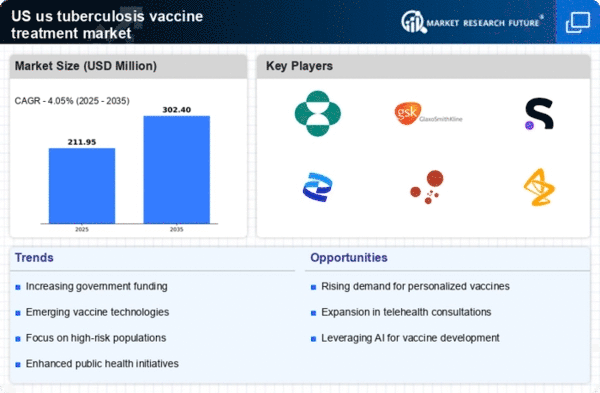
The US Tuberculosis Vaccine Treatment Market is experiencing a notable increase in the incidence of tuberculosis (TB) cases. According to the Centers for Disease Control and Prevention (CDC), there were approximately 9,000 reported cases of TB in the United States in 2022, indicating a concerning trend. This rise in cases necessitates the urgent development and distribution of effective vaccines, thereby driving market growth. The increasing number of drug-resistant TB strains further complicates treatment options, highlighting the need for innovative vaccine solutions. As public health officials prioritize TB control, the demand for vaccines is expected to escalate, creating opportunities for pharmaceutical companies to invest in research and development. Consequently, the rising incidence of TB is a critical driver for the US Tuberculosis Vaccine Treatment Market.
Increased Awareness and Education
Public awareness and education regarding tuberculosis are critical factors influencing the US Tuberculosis Vaccine Treatment Market. Campaigns aimed at educating the public about TB transmission, symptoms, and prevention strategies have gained momentum in recent years. Organizations such as the CDC and various non-profits are actively promoting awareness initiatives, which have led to increased screening and vaccination rates. As individuals become more informed about the risks associated with TB, the demand for vaccines is likely to rise. Furthermore, healthcare providers are increasingly emphasizing the importance of vaccination in preventing TB outbreaks. This heightened awareness not only drives vaccine uptake but also encourages policymakers to prioritize TB vaccination programs. Consequently, increased awareness and education represent a vital driver for the growth of the US Tuberculosis Vaccine Treatment Market.
Advancements in Vaccine Technology
Technological advancements are transforming the landscape of the US Tuberculosis Vaccine Treatment Market. Innovations in vaccine formulation, delivery systems, and adjuvant technologies are enhancing the efficacy and safety profiles of TB vaccines. For example, the development of recombinant vaccines and mRNA technology has shown promise in preclinical studies, potentially leading to more effective TB prevention strategies. These advancements not only improve vaccine performance but also streamline the manufacturing process, making it more cost-effective. As research institutions and biotech companies continue to explore novel approaches to TB vaccination, the market is likely to witness an influx of new products. Thus, the ongoing advancements in vaccine technology serve as a crucial driver for the US Tuberculosis Vaccine Treatment Market, fostering a competitive environment for vaccine developers.
Global Collaboration and Partnerships
The Tuberculosis Vaccine Treatment Industry. Collaborative efforts between governments, non-governmental organizations, and private sector entities are essential for addressing the global TB epidemic. Initiatives such as the Global Fund and the Stop TB Partnership facilitate knowledge sharing, resource allocation, and joint research efforts. These collaborations often lead to the development of innovative vaccine candidates and enhance access to vaccines in underserved populations. In the US, partnerships between academic institutions and pharmaceutical companies are fostering the translation of research into viable vaccine products. As these collaborative efforts continue to expand, they are likely to accelerate the pace of vaccine development and distribution, thereby driving growth in the US Tuberculosis Vaccine Treatment Market.