Rising Prevalence of Chronic Diseases
The rising prevalence of chronic diseases in the US is a significant driver for the stem cell-therapy market. Conditions such as diabetes, cardiovascular diseases, and neurodegenerative disorders are becoming increasingly common, necessitating innovative treatment options. Stem cell therapies offer potential solutions for regenerating damaged tissues and organs, which traditional treatments may not effectively address. According to recent statistics, chronic diseases account for nearly 70% of all deaths in the US, highlighting the urgent need for advanced therapeutic interventions. This growing patient population is likely to propel demand for stem cell therapies, as healthcare providers seek effective alternatives to manage these conditions. Consequently, the stem cell-therapy market is expected to expand as it addresses the healthcare challenges posed by chronic diseases.
Increased Public Awareness and Acceptance
Public awareness and acceptance of stem cell therapies are on the rise, significantly impacting the stem cell-therapy market. Educational campaigns and media coverage have played a crucial role in informing the public about the potential benefits of stem cell treatments. As patients become more informed, they are more likely to seek out these therapies for various health conditions. Surveys indicate that approximately 60% of the population is now aware of stem cell therapies and their applications, compared to just 30% a decade ago. This shift in perception is likely to drive demand, as patients advocate for access to innovative treatments. The growing acceptance of stem cell therapies is expected to foster a more favorable regulatory environment, further enhancing the growth prospects of the stem cell-therapy market.
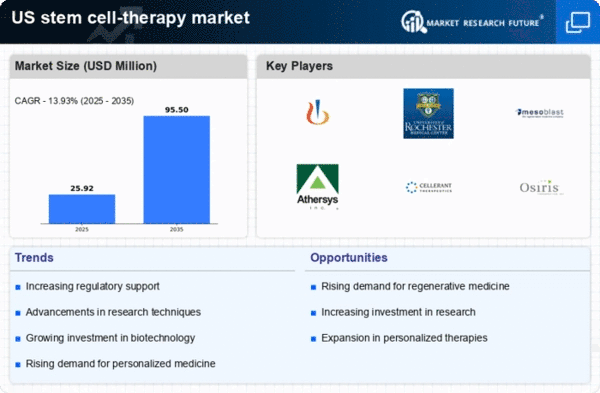
Regulatory Support for Stem Cell Therapies
Regulatory support for stem cell therapies is emerging as a vital driver for the stem cell-therapy market. The US Food and Drug Administration (FDA) has been actively working to establish clear guidelines for the development and approval of stem cell-based treatments. This regulatory framework aims to ensure patient safety while promoting innovation in the field. Recent initiatives have streamlined the approval process for certain stem cell therapies, allowing for faster access to potentially life-saving treatments. As regulatory bodies continue to support the advancement of stem cell research, the market is likely to experience increased activity, with more therapies entering the market. This supportive environment is expected to enhance investor confidence and stimulate further growth in the stem cell-therapy market.
Technological Innovations in Stem Cell Research
The stem cell-therapy market is experiencing a surge in technological innovations that enhance research capabilities and treatment efficacy. Advanced techniques such as CRISPR gene editing and 3D bioprinting are revolutionizing the way stem cells are utilized in therapies. These innovations not only improve the precision of stem cell applications but also expand the range of diseases that can be treated. For instance, the integration of artificial intelligence in stem cell research is streamlining the identification of potential therapies, thereby accelerating the development process. As a result, the market is projected to grow at a CAGR of approximately 10% over the next five years., driven by these technological advancements. The increasing availability of sophisticated tools and methodologies is likely to bolster the overall growth of the stem cell-therapy market.
Investment in Stem Cell Research and Development
Investment in stem cell research and development is a critical driver for the stem cell-therapy market. Both public and private sectors are increasingly allocating funds to explore the therapeutic potential of stem cells. In recent years, venture capital investments in biotechnology firms focusing on stem cell therapies have surged, with funding reaching over $1 billion annually. This influx of capital is facilitating the advancement of clinical trials and the commercialization of new therapies. Furthermore, government grants and initiatives aimed at promoting regenerative medicine are providing additional support for research endeavors. As financial backing continues to grow, the stem cell-therapy market is likely to benefit from accelerated innovation and the introduction of novel treatment options.