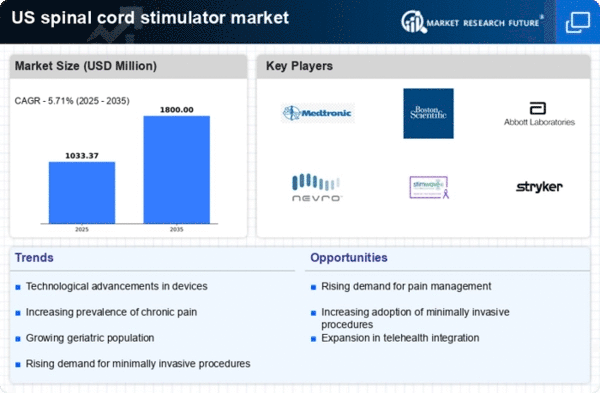
Rising Prevalence of Chronic Pain
The increasing incidence of chronic pain conditions in the US is a primary driver for the spinal cord-stimulator market. Conditions such as neuropathic pain, back pain, and fibromyalgia are becoming more prevalent, affecting millions of individuals. According to recent estimates, approximately 20% of adults in the US experience chronic pain, leading to a growing demand for effective pain management solutions. Spinal cord stimulators offer a minimally invasive option for patients who do not respond to traditional pain relief methods. This trend is likely to continue, as healthcare providers seek innovative treatments to address the needs of this expanding patient population, thereby propelling the spinal cord-stimulator market forward.
Expansion of Reimbursement Policies
The expansion of reimbursement policies for spinal cord stimulators is a crucial driver for the market. Insurance companies and government programs are increasingly recognizing the value of these devices in managing chronic pain. Recent changes in reimbursement guidelines have made spinal cord stimulators more accessible to patients, thereby encouraging their adoption. As reimbursement rates improve, healthcare providers are more likely to recommend these devices as a treatment option. This trend is expected to continue, as stakeholders in the healthcare system work to ensure that patients have access to effective pain management solutions, further stimulating the spinal cord-stimulator market.
Increased Investment in Healthcare Technology
Investment in healthcare technology is witnessing a notable surge, which significantly impacts the spinal cord-stimulator market. With advancements in medical devices and a focus on improving patient outcomes, manufacturers are allocating substantial resources to research and development. The US healthcare system is increasingly adopting innovative technologies, with spending on medical devices projected to reach $208 billion by 2025. This influx of capital fosters the development of more sophisticated spinal cord stimulators, enhancing their efficacy and safety profiles. As healthcare providers embrace these advancements, the spinal cord-stimulator market is poised for growth, driven by the demand for cutting-edge pain management solutions.
Technological Integration with Digital Health Solutions
The integration of spinal cord stimulators with digital health solutions is emerging as a significant driver in the market. The advent of telemedicine and remote monitoring technologies allows for enhanced patient management and follow-up care. Patients can now receive real-time feedback on their spinal cord stimulators, leading to improved treatment outcomes. This technological synergy is likely to attract more patients to consider spinal cord stimulators as a viable option for pain management. As the healthcare landscape evolves, the spinal cord-stimulator market is expected to benefit from this integration, fostering growth and innovation in the field.
Growing Awareness of Alternative Pain Management Solutions
There is a rising awareness among patients and healthcare professionals regarding alternative pain management solutions, which is positively influencing the spinal cord-stimulator market. As individuals seek to avoid opioid dependency and its associated risks, they are increasingly turning to non-pharmacological treatments. Spinal cord stimulators are gaining recognition as a viable option for managing chronic pain, with studies indicating that they can reduce pain levels by up to 70% in some patients. This shift in perception is likely to drive demand for spinal cord stimulators, as more patients and providers explore innovative approaches to pain management.