Rising Demand for Biologics
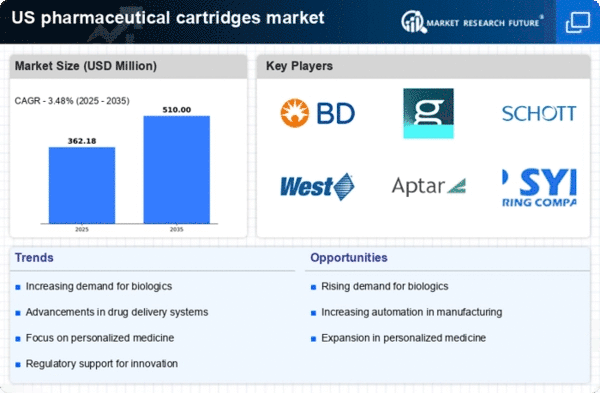
The pharmaceutical cartridges market experiences a notable surge in demand due to the increasing prevalence of biologics. These complex drugs, derived from living organisms, require precise delivery mechanisms, which pharmaceutical cartridges provide. The market for biologics is projected to reach approximately $300 billion by 2025, indicating a robust growth trajectory. As healthcare providers seek efficient and reliable methods for administering biologics, the pharmaceutical cartridges market is poised to benefit significantly. This trend is further supported by the growing number of biologic therapies approved by regulatory bodies, which enhances the need for specialized delivery systems. Consequently, the pharmaceutical cartridges market is likely to expand as manufacturers innovate to meet the specific requirements of biologic formulations.
Shift Towards Home Healthcare
The pharmaceutical cartridges market is influenced by the increasing shift towards home healthcare solutions. As patients prefer receiving treatments in the comfort of their homes, the demand for user-friendly and efficient drug delivery systems rises. Pharmaceutical cartridges facilitate self-administration of medications, which aligns with the growing trend of patient-centric care. The home healthcare market is expected to grow at a CAGR of around 8% through 2025, further driving the need for innovative delivery systems. This shift not only enhances patient compliance but also reduces healthcare costs, making the pharmaceutical cartridges market an essential component of the evolving healthcare landscape. Manufacturers are likely to focus on developing cartridges that cater to the specific needs of home healthcare, thereby expanding their market presence.
Technological Integration in Drug Delivery
The pharmaceutical cartridges market is witnessing a transformation due to the integration of advanced technologies in drug delivery systems. Innovations such as smart cartridges equipped with sensors and connectivity features are enhancing the efficiency and effectiveness of medication administration. These technologies allow for real-time monitoring of dosage and adherence, which is crucial for patient outcomes. The market for smart drug delivery systems is expected to grow significantly, potentially reaching $50 billion by 2025. This technological advancement not only improves patient safety but also provides healthcare professionals with valuable data for better management of treatment plans. As a result, the pharmaceutical cartridges market is likely to see increased investment in research and development to create next-generation delivery systems.
Increased Focus on Chronic Disease Management
The pharmaceutical cartridges market is significantly impacted by the rising focus on chronic disease management. With chronic diseases such as diabetes and cardiovascular conditions on the rise, there is an increasing need for effective drug delivery systems. Pharmaceutical cartridges offer precise dosing and ease of use, which are critical for managing these long-term conditions. The chronic disease management market is projected to reach $200 billion by 2025, indicating a substantial opportunity for the pharmaceutical cartridges market. As healthcare providers emphasize the importance of adherence to treatment regimens, the demand for reliable delivery systems is likely to grow. This trend suggests that the pharmaceutical cartridges market will continue to evolve, with manufacturers developing solutions tailored to the needs of chronic disease patients.
Growing Regulatory Support for Advanced Delivery Systems
The pharmaceutical cartridges market benefits from growing regulatory support aimed at advancing drug delivery systems. Regulatory agencies are increasingly recognizing the importance of innovative delivery methods in improving patient outcomes. This support is reflected in streamlined approval processes for new delivery technologies, which encourages manufacturers to invest in research and development. The market for advanced drug delivery systems is projected to grow at a CAGR of approximately 7% through 2025, driven by favorable regulatory environments. As regulations evolve to accommodate new technologies, the pharmaceutical cartridges market is likely to expand, with manufacturers developing compliant and effective delivery solutions that meet the needs of healthcare providers and patients alike.