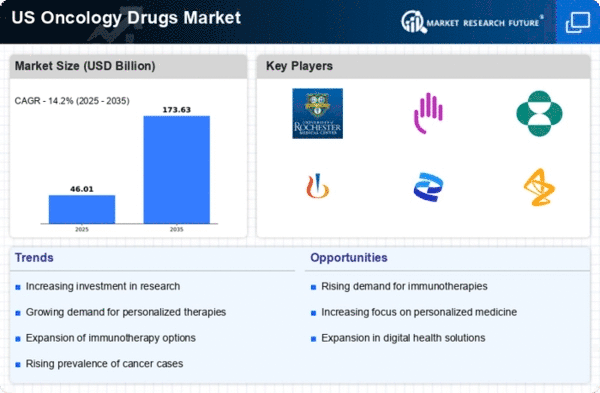
Top Industry Leaders in the US Oncology Drugs Market

Latest U.S. Oncology Drugs Companies Update
Eli Lilly's Jaypirca® (pirtobrutinib) receives FDA approval for the treatment of mantle cell lymphoma, offering a targeted therapy option with promising efficacy and improved tolerability.
BMS's Opdivo® (nivolumab) with Yervoy® (ipilimumab) combination secures FDA approval for the first-line treatment of non-small cell lung cancer (NSCLC) with high PD-L1 expression, extending options for early-stage patients.
Seagen's Tukysa® (tucatinib) in combination with Xeloda® (capecitabine) and Herceptin® (trastuzumab) receives FDA Breakthrough Therapy Designation for HER2-positive metastatic colorectal cancer, potentially accelerating its pathway to market.
Pfizer acquires Global Blood Therapeutics for USD 5.4 billion, gaining access to GBT's portfolio of hemophilia and sickle cell disease treatments, strengthening its presence in rare disease therapeutics.
Merck & Co. enters into a definitive agreement to acquire Veloxis Pharmaceuticals for USD 2.75 billion, acquiring Veloxis's pipeline of oncology candidates, including VX-11e, a PARP inhibitor with potential for pancreatic cancer treatment.
AbbVie and Genmab expand their strategic partnership, focusing on the development and commercialization of bispecific T-cell engagers (BiTEs) for various cancer types, leveraging their combined expertise in antibody engineering and clinical development.
List of U.S. Oncology Drugs Key companies in the market
-
GlaxoSmithKline PIc.
-
Novartis AG
-
Merck & Co. Inc.
-
Eli Lilly and Company
-
Amgen Inc.
-
Bayer
-
Celgene Corporation
-
Johnson & Johnson
-
Pfizer Inc.