Rising Incidence of Epilepsy
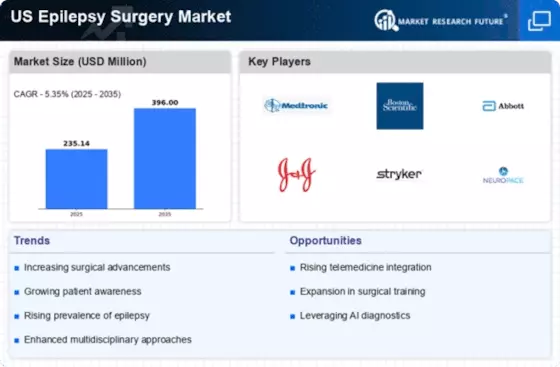
The US Epilepsy Surgery Market is experiencing growth due to the rising incidence of epilepsy across the nation. According to the Centers for Disease Control and Prevention (CDC), approximately 1.2% of the US population is affected by epilepsy, translating to nearly 3 million individuals. This increasing prevalence necessitates advanced treatment options, including surgical interventions for drug-resistant epilepsy. As more patients seek effective solutions, the demand for epilepsy surgery is likely to rise, driving market expansion. Furthermore, the growing recognition of epilepsy as a significant public health issue has prompted healthcare providers to explore surgical options, thereby enhancing the overall landscape of the US Epilepsy Surgery Market.
Supportive Regulatory Framework
The US Epilepsy Surgery Market benefits from a supportive regulatory framework that encourages innovation and accessibility. The Food and Drug Administration (FDA) has streamlined the approval process for new surgical devices and techniques, facilitating quicker access to cutting-edge treatments for patients. Additionally, the establishment of guidelines for epilepsy surgery by professional organizations has standardized practices, ensuring that patients receive optimal care. This regulatory support not only enhances the safety and efficacy of surgical interventions but also fosters an environment conducive to market growth. As more surgical options become available, the US Epilepsy Surgery Market is poised for further expansion.
Advancements in Neuroimaging Techniques
Innovations in neuroimaging technologies are playing a pivotal role in the US Epilepsy Surgery Market. Techniques such as functional MRI and PET scans have significantly improved the ability to localize epileptic foci, which is crucial for surgical planning. Enhanced imaging capabilities allow for more precise identification of seizure origins, thereby increasing the success rates of surgical interventions. As a result, the integration of advanced neuroimaging into clinical practice is likely to bolster the confidence of both patients and healthcare providers in pursuing epilepsy surgery. This trend is expected to contribute positively to the growth trajectory of the US Epilepsy Surgery Market.
Growing Investment in Epilepsy Research
Investment in epilepsy research is a significant driver of the US Epilepsy Surgery Market. Public and private funding initiatives are increasingly directed towards understanding the underlying mechanisms of epilepsy and developing innovative surgical techniques. For instance, the National Institutes of Health (NIH) allocates substantial resources to epilepsy research, which has led to breakthroughs in surgical approaches. This influx of funding not only supports clinical trials but also encourages collaboration among researchers, healthcare providers, and industry stakeholders. As research continues to advance, the US Epilepsy Surgery Market is likely to benefit from new insights and improved surgical options.
Increased Patient Advocacy and Education
The US Epilepsy Surgery Market is witnessing a surge in patient advocacy and education efforts aimed at raising awareness about surgical options. Organizations dedicated to epilepsy are actively promoting the benefits of surgery for individuals with drug-resistant epilepsy, thereby empowering patients to make informed decisions about their treatment. Educational campaigns and support groups are instrumental in disseminating information about the potential success of epilepsy surgery, which may lead to increased patient referrals for surgical evaluation. As awareness grows, the US Epilepsy Surgery Market is expected to experience heightened demand for surgical interventions, ultimately improving patient outcomes.