Rising Incidence of Epilepsy
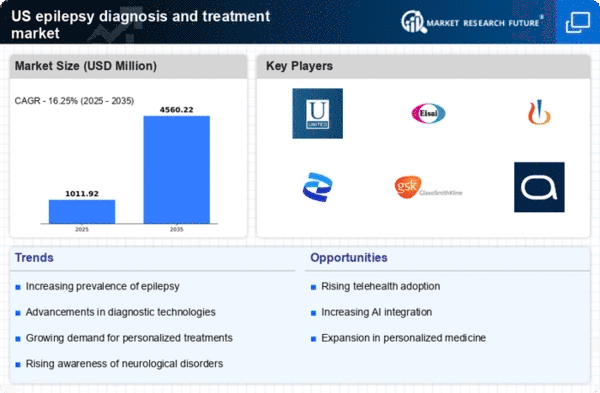
The increasing incidence of epilepsy in the US is a crucial driver for the epilepsy diagnosis-treatment market. Recent estimates suggest that approximately 3.4 million people in the US are living with epilepsy, with around 150,000 new cases diagnosed annually. This growing patient population necessitates enhanced diagnostic tools and treatment options, thereby propelling market growth. As awareness of epilepsy expands, healthcare providers are more likely to seek advanced diagnostic methods and effective treatment regimens. The rising incidence also places a burden on healthcare systems, prompting investments in research and development to improve patient outcomes. Consequently, the epilepsy diagnosis-treatment market is expected to experience significant growth as stakeholders respond to the increasing demand for effective management solutions.
Advancements in Neurotechnology
Technological innovations in neurotechnology are transforming the landscape of the epilepsy diagnosis-treatment market. Developments in neuroimaging techniques, such as functional MRI and PET scans, enable more accurate diagnosis and localization of seizure foci. Additionally, wearable devices that monitor brain activity in real-time are gaining traction, providing valuable data for both patients and healthcare providers. The integration of artificial intelligence in analyzing EEG data is also emerging, potentially enhancing diagnostic accuracy and treatment personalization. As these technologies become more accessible, they are likely to drive market growth by improving patient management and outcomes. The epilepsy diagnosis-treatment market stands to benefit from these advancements, as they facilitate timely interventions and tailored therapeutic approaches.
Growing Demand for Telemedicine
The rise of telemedicine is reshaping the epilepsy diagnosis-treatment market by providing patients with greater access to care. Telehealth services allow individuals to consult with specialists remotely, which is particularly beneficial for those living in rural or underserved areas. This shift towards virtual consultations has been accelerated by the need for accessible healthcare solutions. Studies indicate that telemedicine can improve patient adherence to treatment plans and enhance monitoring of seizure activity. As healthcare providers increasingly adopt telehealth platforms, the epilepsy diagnosis-treatment market is expected to grow, driven by the demand for convenient and efficient care options. This trend may also lead to improved patient outcomes and satisfaction.
Increased Focus on Mental Health
The growing recognition of the link between epilepsy and mental health issues is influencing the epilepsy diagnosis-treatment market. Many individuals with epilepsy experience comorbid conditions such as anxiety and depression, which can complicate treatment and management. As mental health awareness rises, healthcare providers are more likely to adopt holistic approaches that address both neurological and psychological aspects of care. This shift may lead to the development of integrated treatment plans that encompass medication, therapy, and lifestyle modifications. Consequently, the epilepsy diagnosis-treatment market is poised for growth as it adapts to the evolving understanding of the interplay between epilepsy and mental health, ultimately improving patient care.
Government Initiatives and Funding
Government initiatives aimed at improving epilepsy care significantly influence the epilepsy diagnosis-treatment market. Various federal and state programs are designed to enhance research funding, public awareness, and access to treatment. For instance, the National Institutes of Health (NIH) allocates substantial resources to epilepsy research, which fosters innovation in diagnostic and therapeutic methods. Furthermore, public health campaigns aimed at educating the population about epilepsy contribute to increased diagnosis rates and treatment uptake. These initiatives not only support the development of new technologies but also ensure that patients receive timely and effective care. As a result, the epilepsy diagnosis-treatment market is likely to expand in response to these supportive governmental efforts.