Increased Focus on Preventive Healthcare
The shift towards preventive healthcare is influencing the drug device-combination market. As healthcare providers and patients alike recognize the importance of early intervention, there is a growing demand for products that facilitate preventive measures. Drug device combinations that enable early detection and management of health conditions are becoming increasingly popular. This trend is supported by initiatives aimed at reducing healthcare costs and improving population health. The market is likely to benefit from this focus, as products that integrate diagnostics with therapeutics can play a crucial role in preventive care strategies.
Regulatory Support for Innovative Therapies
Regulatory bodies in the US are increasingly supportive of innovative therapies, which is positively impacting the drug device-combination market. Initiatives aimed at expediting the approval process for novel products are encouraging companies to invest in the development of combination therapies. The FDA's 21st Century Cures Act and other regulatory frameworks are designed to facilitate faster access to market for breakthrough products. This supportive environment is likely to foster innovation and encourage the introduction of new drug device combinations that address unmet medical needs, ultimately benefiting patients and healthcare providers.
Rising Demand for Chronic Disease Management
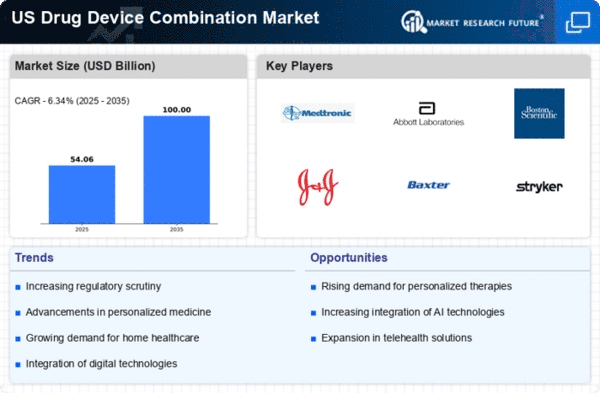
The increasing prevalence of chronic diseases in the US is driving the drug device-combination market. As the population ages, conditions such as diabetes, cardiovascular diseases, and respiratory disorders are becoming more common. This trend necessitates innovative solutions that combine therapeutic drugs with delivery devices, enhancing patient adherence and outcomes. According to recent estimates, chronic diseases account for approximately 75% of healthcare spending in the US, highlighting the urgent need for effective management strategies. The drug device-combination market is positioned to address these challenges by offering integrated solutions that streamline treatment processes and improve patient quality of life.
Growing Investment in Research and Development
Investment in research and development (R&D) is a key driver of the drug device-combination market. Pharmaceutical and medical device companies are allocating substantial resources to innovate and develop new products that meet evolving healthcare needs. This trend is evident in the increasing number of collaborations between biotech firms and established pharmaceutical companies, aimed at leveraging complementary expertise. In 2025, R&D spending in the healthcare sector is expected to exceed $200 billion, underscoring the commitment to advancing drug device combinations that enhance treatment options and patient outcomes.
Technological Advancements in Drug Delivery Systems
Technological innovations are significantly impacting the drug device-combination market. Advances in materials science, microelectronics, and biotechnology are enabling the development of sophisticated drug delivery systems. These systems can provide controlled release, targeted delivery, and real-time monitoring of therapeutic effects. For instance, smart inhalers and implantable devices are gaining traction, offering enhanced patient engagement and adherence. The market for such devices is projected to grow at a CAGR of around 10% over the next five years, indicating a robust demand for innovative solutions that improve treatment efficacy and patient outcomes.