Increased Investment in R&D
Increased investment in research and development (R&D) is a pivotal driver of the commercial pharmaceutical-analytics market. Pharmaceutical companies are allocating substantial resources to R&D to foster innovation and expedite the development of new therapies. In 2025, R&D spending in the pharmaceutical sector is projected to exceed $200 billion, with analytics playing a crucial role in optimizing these investments. By utilizing analytics to streamline R&D processes, companies can enhance their ability to identify promising drug candidates and reduce development costs. This focus on R&D not only propels advancements in the commercial pharmaceutical-analytics market but also contributes to improved patient outcomes.
Integration of Advanced Technologies
The integration of advanced technologies such as cloud computing and artificial intelligence is transforming the commercial pharmaceutical-analytics market. These technologies facilitate the processing of vast amounts of data, enabling pharmaceutical companies to derive actionable insights more efficiently. For instance, cloud-based analytics solutions are expected to account for over 40% of the market by 2026, as they offer scalability and flexibility. Furthermore, the use of AI algorithms in data analysis can enhance predictive modeling, allowing companies to anticipate market shifts and patient behaviors. This technological evolution is likely to enhance operational efficiency and foster innovation within the commercial pharmaceutical-analytics market.
Rising Demand for Data-Driven Insights
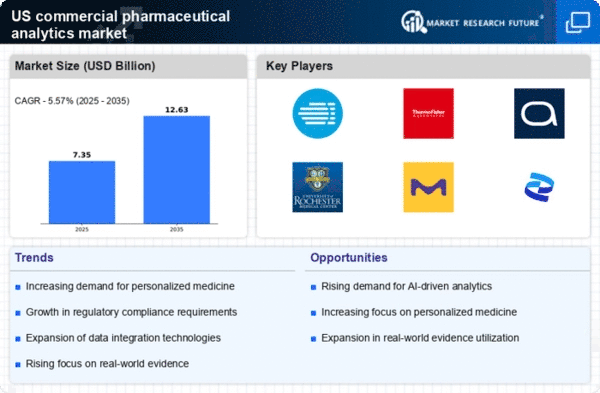
The commercial pharmaceutical-analytics market is experiencing a notable surge in demand for data-driven insights. Pharmaceutical companies are increasingly relying on analytics to enhance decision-making processes, optimize drug development, and improve patient outcomes. This trend is underscored by the fact that analytics can reduce the time to market for new drugs by up to 30%. As a result, organizations are investing heavily in advanced analytics solutions, which are projected to grow at a CAGR of 12% through 2027. The ability to leverage big data analytics allows companies to identify market trends and understand patient needs. This understanding enables them to tailor their strategies accordingly, thereby driving growth in the commercial pharmaceutical-analytics market.
Regulatory Landscape and Compliance Needs
The evolving regulatory landscape is a critical driver for the commercial pharmaceutical-analytics market. As regulatory bodies impose stricter guidelines on data management and reporting, pharmaceutical companies are compelled to adopt robust analytics solutions to ensure compliance. Regulatory compliance analytics is expected to reach $3 billion by 2025, reflecting the increasing need for transparency and accountability in drug development processes. Companies that effectively leverage analytics to navigate regulatory requirements can mitigate risks and enhance their market positioning. Thus, the regulatory landscape significantly influences the growth trajectory of the commercial pharmaceutical-analytics market.
Growing Emphasis on Patient-Centric Approaches
There is a growing emphasis on patient-centric approaches within the commercial pharmaceutical-analytics market. Pharmaceutical companies are increasingly focusing on understanding patient experiences and outcomes to develop more effective therapies. This shift is reflected in the rising investment in patient-reported outcomes (PRO) analytics, which is projected to grow by 15% annually. By utilizing analytics to gather and analyze patient data, companies can tailor their products to meet specific patient needs, thereby improving adherence and satisfaction. This patient-centric focus not only enhances the therapeutic value but also drives competitive advantage in the commercial pharmaceutical-analytics market.