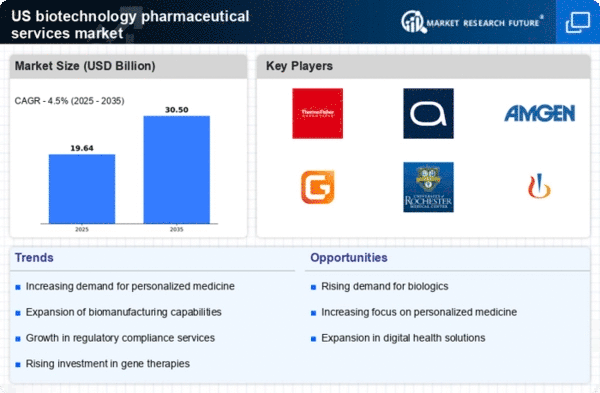
Rising Demand for Biologics
The The biotechnology pharmaceutical services market is seeing a notable increase in demand for biologics., which are complex drugs derived from living organisms. This trend is driven by the growing prevalence of chronic diseases and the need for innovative therapies. According to recent data, biologics accounted for approximately 40% of total pharmaceutical sales in the US, highlighting their significance in the market. As healthcare providers and patients seek more effective treatment options, the biotechnology pharmaceutical-services-outsources market is likely to expand further, necessitating enhanced outsourcing services to meet production and regulatory requirements.
Increased Focus on Cost Efficiency
Cost efficiency remains a critical driver in the biotechnology pharmaceutical-services-outsources market. Companies are increasingly looking to optimize their operations by outsourcing non-core functions, which allows them to focus on their primary competencies. By leveraging the expertise of specialized service providers, biotechnology firms can reduce operational costs by up to 30%. This trend is particularly relevant in the context of rising R&D expenses and the need for sustainable business models. As a result, the demand for outsourcing services is expected to grow, further shaping the landscape of the biotechnology pharmaceutical-services-outsources market.
Regulatory Support and Streamlining
The biotechnology pharmaceutical-services-outsources market benefits from a favorable regulatory environment that aims to streamline the approval process for new therapies. Recent initiatives by the FDA have focused on expediting the review of biologics and biosimilars, which could potentially reduce time-to-market for new products. This regulatory support is crucial for biotechnology companies seeking to navigate complex compliance landscapes. As a result, outsourcing partners are increasingly sought after to assist with regulatory submissions and quality assurance, thereby enhancing the overall efficiency of the biotechnology pharmaceutical-services-outsources market.
Growing Importance of Data Analytics
The role of data analytics in the biotechnology pharmaceutical-services-outsources market is becoming increasingly prominent. Companies are harnessing big data to gain insights into patient outcomes, treatment efficacy, and market trends. This analytical approach enables firms to make informed decisions regarding drug development and marketing strategies. In 2025, it is estimated that the market for data analytics in the pharmaceutical sector will exceed $20 billion in the US. As biotechnology companies seek to leverage data for competitive advantage, the demand for outsourcing services that specialize in data management and analytics is likely to rise, influencing the overall dynamics of the biotechnology pharmaceutical-services-outsources market.
Advancements in Research and Development
Innovations in research and development (R&D) are propelling the biotechnology pharmaceutical-services-outsources market forward. The integration of cutting-edge technologies, such as CRISPR and gene editing, is facilitating the discovery of new therapeutic targets and accelerating drug development timelines. In 2025, R&D spending in the biotechnology sector is projected to reach $100 billion in the US, indicating a robust investment landscape. This influx of funding is expected to enhance the capabilities of outsourcing partners, enabling them to provide specialized services that cater to the evolving needs of biotechnology firms.