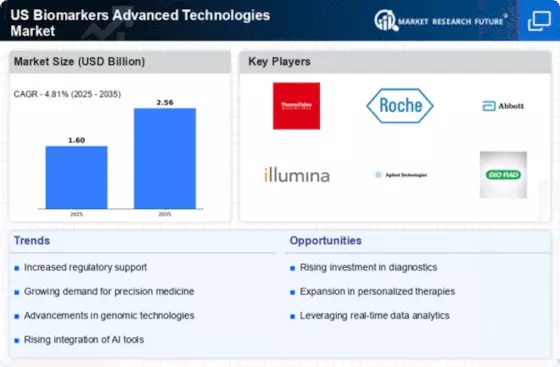
Regulatory Framework Enhancements
The regulatory landscape surrounding the US Biomarkers Advanced Technologies Market is evolving, with enhancements aimed at expediting the approval process for new biomarker-based diagnostics. The Food and Drug Administration (FDA) has introduced initiatives to streamline the review of biomarker applications, thereby encouraging innovation. For instance, the FDA's Biomarker Qualification Program allows for the assessment of biomarkers in drug development, which could significantly reduce the time to market for new diagnostic tools. This supportive regulatory environment is likely to attract more companies to invest in biomarker research and development, fostering competition and innovation. As regulatory pathways become clearer and more efficient, the market is expected to expand, providing patients with access to cutting-edge diagnostic solutions.
Advancements in Genomic Technologies
The US Biomarkers Advanced Technologies Market is significantly influenced by rapid advancements in genomic technologies. Innovations in next-generation sequencing (NGS) and CRISPR gene editing are revolutionizing the way biomarkers are identified and utilized in clinical settings. The market for NGS alone is projected to reach USD 10 billion by 2026, driven by its applications in personalized medicine and targeted therapies. These technologies enable researchers to analyze vast amounts of genetic data, facilitating the discovery of novel biomarkers that can predict disease susceptibility and treatment response. As the cost of genomic sequencing continues to decline, more healthcare institutions are likely to adopt these technologies, thereby propelling the growth of the biomarkers market. This trend underscores the importance of integrating cutting-edge genomic tools into the biomarker discovery process.
Rising Awareness of Precision Medicine
The growing awareness of precision medicine is a pivotal driver for the US Biomarkers Advanced Technologies Market. As healthcare shifts towards more personalized approaches, the role of biomarkers in tailoring treatments to individual patients becomes increasingly critical. Educational campaigns and initiatives by healthcare organizations are enhancing public understanding of the benefits of precision medicine, which is projected to reach a market size of USD 100 billion by 2026. This shift is prompting healthcare providers to adopt biomarker testing as a standard practice, thereby increasing demand for advanced technologies that facilitate such testing. The emphasis on individualized treatment plans is likely to propel the growth of the biomarkers market, as stakeholders recognize the value of integrating biomarker data into clinical decision-making.
Growing Demand for Early Disease Detection
The US Biomarkers Advanced Technologies Market is experiencing a notable surge in demand for early disease detection methods. This trend is largely driven by an increasing prevalence of chronic diseases, such as cancer and diabetes, which necessitate timely diagnosis for effective treatment. According to the National Cancer Institute, approximately 1.9 million new cancer cases are expected in the US in 2026, highlighting the urgent need for advanced diagnostic tools. Biomarkers play a crucial role in identifying disease at its nascent stages, thereby improving patient outcomes. The integration of advanced technologies, such as liquid biopsies and genomic profiling, is expected to enhance the accuracy and efficiency of these diagnostic processes. Consequently, the market is likely to expand as healthcare providers and patients alike prioritize early detection strategies.
Increased Investment in Research and Development
Investment in research and development (R&D) within the US Biomarkers Advanced Technologies Market is on the rise, reflecting a commitment to innovation and improved healthcare outcomes. Government funding, alongside private sector investments, is fostering an environment conducive to biomarker research. The National Institutes of Health (NIH) allocated over USD 41 billion for biomedical research in 2025, a portion of which is directed towards biomarker studies. This influx of capital is likely to accelerate the development of novel biomarkers and associated technologies, enhancing diagnostic capabilities and therapeutic strategies. Furthermore, collaborations between academic institutions and biotechnology firms are becoming increasingly common, facilitating knowledge transfer and resource sharing. As a result, the market is poised for substantial growth, driven by a robust pipeline of innovative biomarker technologies.