Increased Research Funding
The surge in research funding dedicated to Alagille Syndrome is a critical driver for the US Alagille Syndrome Market. Government and private organizations are increasingly investing in research initiatives aimed at understanding the genetic and environmental factors contributing to the syndrome. This influx of funding is likely to accelerate the development of innovative therapies and treatment modalities. For instance, the National Institutes of Health has allocated resources to support studies focused on the pathophysiology of Alagille Syndrome. As new findings emerge, they may lead to the introduction of novel therapeutic options, thereby enhancing the overall market landscape for the US Alagille Syndrome Market.
Emergence of Targeted Therapies
The emergence of targeted therapies for Alagille Syndrome represents a transformative driver for the US Alagille Syndrome Market. Recent advancements in pharmacological research have led to the development of therapies that specifically address the underlying genetic causes of the syndrome. These targeted treatments are designed to improve liver function and overall health outcomes for patients. As clinical trials continue to demonstrate the efficacy of these therapies, there is a growing anticipation for their approval and subsequent market entry. The potential for these innovative treatments to change the standard of care for Alagille Syndrome patients is likely to stimulate market growth and attract investment in the US Alagille Syndrome Market.
Growing Awareness and Education
The growing awareness and education surrounding Alagille Syndrome are pivotal in shaping the US Alagille Syndrome Market. Increased efforts by healthcare organizations and advocacy groups to educate both the public and healthcare professionals about the syndrome are leading to more timely diagnoses. This heightened awareness is likely to result in a greater number of patients seeking medical attention, which in turn drives demand for specialized care and treatment options. Additionally, educational campaigns are fostering a better understanding of the condition, encouraging families to pursue genetic counseling and testing. This trend is expected to contribute positively to the growth of the US Alagille Syndrome Market.
Rising Prevalence of Alagille Syndrome
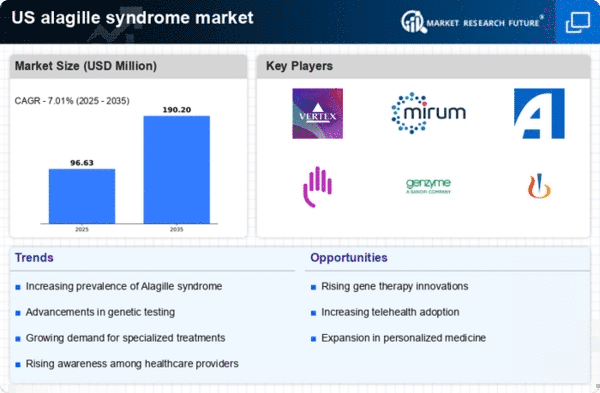
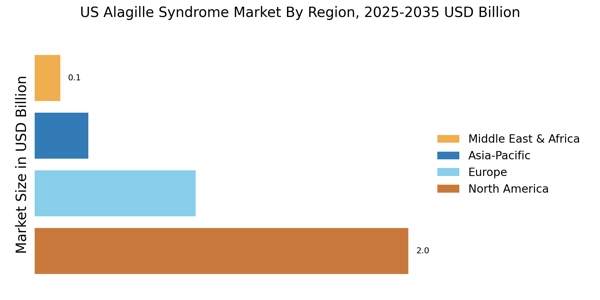
The increasing prevalence of Alagille Syndrome in the United States is a notable driver for the US Alagille Syndrome Market. Recent estimates suggest that the incidence of this genetic disorder ranges from 1 in 30,000 to 1 in 50,000 live births. As awareness of the condition grows, more patients are being diagnosed, which in turn drives demand for specialized treatments and healthcare services. This rising prevalence is likely to lead to an increase in healthcare expenditures related to the management of Alagille Syndrome, thereby expanding the market. Furthermore, the need for comprehensive care strategies, including liver transplantation and ongoing medical management, underscores the importance of addressing this condition within the US Alagille Syndrome Market.
Advancements in Diagnostic Technologies
Technological advancements in diagnostic tools are significantly influencing the US Alagille Syndrome Market. Enhanced imaging techniques and genetic testing have improved the accuracy of diagnosing Alagille Syndrome, allowing for earlier detection and intervention. The introduction of next-generation sequencing has made it possible to identify genetic mutations associated with the syndrome more efficiently. As a result, healthcare providers can offer tailored treatment plans, which may lead to better patient outcomes. The growing adoption of these advanced diagnostic technologies is expected to increase the number of diagnosed cases, thereby expanding the market for related therapeutic options and healthcare services in the US Alagille Syndrome Market.