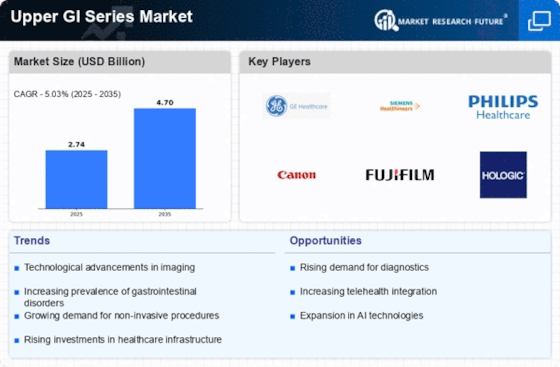
Top Industry Leaders in the Upper GI Series Market

Motus GI Receives FDA Clearance and Successfully Completes First Procedure for Pure-Vu® EVS Gastro:A significant development came in October 2023 when Motus GI, a company specializing in endoscopic visualization technology, received FDA clearance for its Pure-Vu® EVS Gastro system.
This innovative device is designed to improve visualization during upper GI procedures, particularly for addressing upper GI bleeds, a critical area with unmet needs.
Shortly after receiving clearance, Motus GI also announced the successful completion of the first patient procedure using Pure-Vu® EVS Gastro, marking a crucial milestone in bringing this technology to market.
Growing Adoption of AI-powered Solutions for Upper GI Analysis:Artificial intelligence (AI) is making waves in the medical field, and the Upper GI Series Market is no exception. Companies like GE Healthcare and Aidoc are developing AI algorithms that can analyze X-rays and other imaging data from upper GI series to detect abnormalities with greater accuracy and efficiency.
- GastroIntestinal Specialists LLC. (UK)
- Eisai Co. Ltd (Japan)
- Cadila Pharmaceuticals Limited (India)
- Purdue Pharma L.P. (US)
- Alfa Wassermann SPA (Germany)
- Novadaq Technologies Inc. (Canada)
- AstraZeneca (UK)
- Ironwood Pharmaceuticals Inc. (US)