Supportive Regulatory Environment
A supportive regulatory environment is fostering growth in the rna based-therapeutics market. The UK regulatory agencies are actively working to streamline the approval processes for RNA-based therapies, thereby encouraging innovation and investment in this sector. Initiatives aimed at expediting clinical trials and providing clear guidelines for the development of RNA therapeutics are enhancing the attractiveness of this market for investors and developers alike. This regulatory support is crucial for bringing new therapies to market more efficiently, which is expected to contribute to a projected market growth of approximately 12% annually over the next five years. The proactive stance of regulatory bodies is likely to bolster confidence among stakeholders in the rna based-therapeutics market.
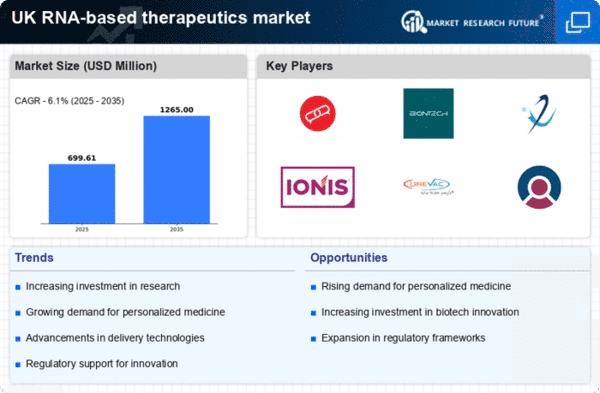
Growing Demand for Personalized Medicine
The RNA-based therapeutics market is experiencing a notable surge in demand for personalized medicine, driven by advancements in genomics and biotechnology. This shift towards tailored treatments is reshaping the landscape of healthcare in the UK. As patients increasingly seek therapies that are specifically designed to address their unique genetic profiles, the rna based-therapeutics market is positioned to benefit significantly. The market is projected to reach approximately £2 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 15%. This growth is indicative of the increasing recognition of the potential of rna-based therapies to provide more effective and targeted treatment options, thereby enhancing patient outcomes and satisfaction.
Increasing Prevalence of Genetic Disorders
The rising incidence of genetic disorders in the UK is significantly influencing the rna based-therapeutics market. Conditions such as cystic fibrosis, muscular dystrophy, and various rare diseases are becoming more prevalent, necessitating innovative treatment solutions. RNA-based therapies offer promising avenues for addressing these genetic challenges, as they can directly target the underlying genetic mutations. The UK government has recognized this need and is investing in initiatives to support the development of RNA therapeutics. This focus on addressing genetic disorders is likely to propel the market forward, with projections indicating a potential market size of £1.8 billion by 2027, driven by the urgent demand for effective therapies.
Rising Investment from Biopharmaceutical Companies
The rna based-therapeutics market is witnessing a surge in investment from biopharmaceutical companies, which is driving innovation and development in this field. As these companies recognize the potential of RNA-based therapies to address unmet medical needs, they are allocating substantial resources towards research and development. This influx of capital is facilitating the advancement of novel RNA therapeutics, including mRNA vaccines and gene editing technologies. The UK is becoming a hub for such investments, with estimates suggesting that funding in this sector could exceed £1 billion by 2025. This trend indicates a strong belief in the future of RNA therapeutics, which is likely to enhance the overall market landscape.
Technological Advancements in RNA Delivery Systems
Technological innovations in RNA delivery systems are playing a crucial role in the expansion of the rna based-therapeutics market. Enhanced delivery mechanisms, such as lipid nanoparticles and viral vectors, are improving the efficacy and safety of RNA therapies. These advancements facilitate the precise targeting of diseased cells while minimizing off-target effects, which is essential for the successful application of RNA therapeutics. The UK is witnessing a rise in research initiatives focused on optimizing these delivery systems, which could potentially lead to more effective treatments for various conditions, including genetic disorders and cancers. As a result, the market is expected to grow at a robust pace, with estimates suggesting a valuation of £1.5 billion by 2025.