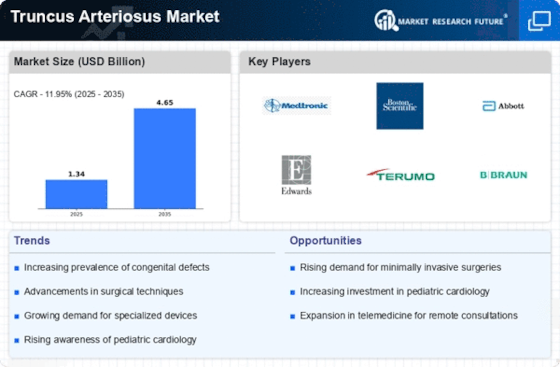
Top Industry Leaders in the Truncus Arteriosus Market

Latest Truncus Arteriosus Companies Update
Abbott Received FDA approval to initiate a first-in-human clinical trial evaluating its Amplatzer Amulet™ Occluder Device for transcatheter closure of the aortopulmonary connection in patients with Truncus Arteriosus. This minimally invasive approach could offer a less risky alternative to traditional open-heart surgery for TA repair.
Sapiens Announced positive preliminary results from its ongoing clinical trial assessing the safety and efficacy of its FORMA™ device for treating TA. The FORMA device aims to reconstruct the aortic arch and separate the pulmonary artery from the aorta, potentially offering a more definitive solution compared to other interventions.
The Truncus Arteriosus Research Consortium (TARC) collaborative group of researchers and clinicians is actively conducting studies to improve the understanding and treatment of TA. Recent efforts include developing standardized outcome measures and identifying genetic factors associated with the condition.
The American Heart Association (AHA) The AHA provides resources and support for families affected by TA and funds research aimed at improving diagnosis, treatment, and long-term outcomes for patients.
List of Truncus Arteriosus Key Companies in the Market
- Boston Scientific Corporation (U.S.)
- Coloplast (Denmark)
- Dispocard GmbH (Germany)
- Jude Medical (U.S.)
- Abbott Laboratories (U.S.)
- Johnson & Johnson (U.S.)
- Teleflex Incorporated (U.S.).
- Smiths Medical (U.S.)
- Edwards Life Sciences Corporation (U.S.)
- Medtronic Inc (U.S.)
- Rochester Medical Corporation (U.S)
- Maquet Medical India Private Limited (India)
- Terumo Medical Corporation (Japan)
- AstraZeneca plc.
- Pfizer Inc.
- Novartis AG
- Merck & Co. Inc.
- Bristol-Myers Squibb Company
- Bayer AG
- Sanofi
- Boehringer Ingelheim GmbH
- Hoffmann-La Roche Ltd
- Gilead Sciences Inc.
- Astellas Pharma Inc.
- Eli Lilly and Company
- Otsuka Holdings Co. Ltd.
- Takeda Pharmaceutical Company Limited.