Market Analysis
In-depth Analysis of Transcatheter Embolization and Occlusion Devices Market Industry Landscape
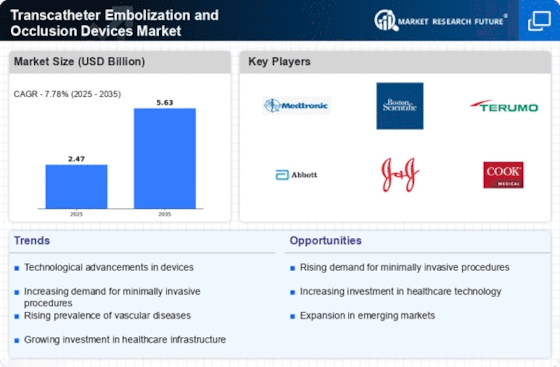
The rising prevalence levels of cardiovascular diseases, aneurysms among other vascular disorders have fueled demand for these products. In addition to encouraging patients to seek alternative methods which are non-invasive compared to traditional surgeries; transcatheter embolization has become popular in the field of vascular intervention.
Technological advancements define the dynamics shaping the transcatheter embolization and occlusion devices market today. They have contributed greatly towards enhancing safety, effectiveness as well as accuracy in vascular interventions through device design innovations i.e., biomaterials used as delivery systems among others. As a result there is growth within these therapeutic options hence more treatment alternatives available for interventional radiologists due to bioresorbable embolic agents being made plus developmental catheters manufactured specifically for this purpose have been refined over time. T
The market dynamics are further influenced by the increasing preference for outpatient and ambulatory procedures. Less invasive interventions in line with patients’ desires for shorter recuperation periods, less hospitalizations and reduced procedural risks have made transcatheter embolization and occlusion devices popular among people. Consequently, these advantages make them pertinent to the market due to the fact that most of them are done on an outpatient basis which results in an increase in this trend. In addition, there is potential for saving costs linked to outpatient treatments especially within healthcare systems which aim at achieving more efficiency.
However challenges within the market dynamics include involvement of complications associated with some procedures complexity and learning curve when using new technologies. Despite being minimally invasive solutions provided by transcatheter embolization and occlusion devices; certain complicated procedures may necessitate special trainings as well as experience. Therefore, continuous education about these products poses a challenge towards their adoption by many health care providers.
Regulatory considerations have significant impacts on the market dynamics of transcatheter embolization & occlusion devices manufacturers. As such, stringent regulatory conditions on product approval as well as entry into the markets dictate developmental timelines and strategies used by manufacturers alike. Thus, fulfilling regulatory standards including safety together with efficacy evaluation becomes important if patient welfare is to be ensured along with successful marketing campaign for these products.
Transcatheter embolization and occlusion devices market dynamics vary geographically due to regional healthcare needs, disease prevalence, and economic factors. Developed regions with advanced healthcare infrastructures and higher burden of cardiovascular diseases tend to have these devices at a high adoption rate. However, increased adoption is being witnessed within emerging economies fueled by increasing cases of vascular disorders as well as expansion of interventional radiology capacities.
















Leave a Comment