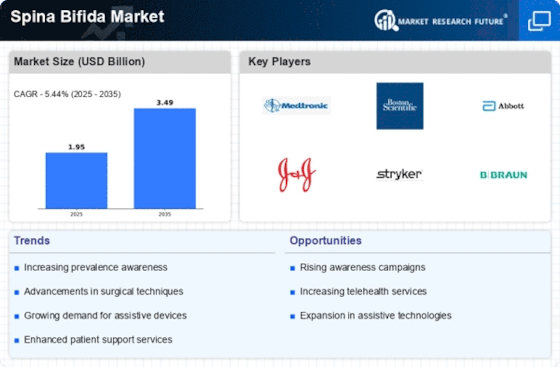
Top Industry Leaders in the Spina Bifida Market

Novartis receives FDA approval for Zolgensma (onasemnogene abeparvovec), a gene therapy for spinal muscular atrophy (SMA) that can also benefit individuals with Spina Bifida Type 1. This groundbreaking therapy offers the potential for significant improvement in motor function and quality of life.
Pfizer launches Vimizim (elosulfase alfa), a treatment for Mucopolysaccharidosis (MPS) VI, a rare lysosomal storage disorder that can sometimes be associated with Spina Bifida. This approval expands treatment options for individuals with MPS VI and related complications.
Development of advanced surgical devices and implants for improved management of Spina Bifida-related orthopedic complications, such as scoliosis and hip dislocation.
Boston Scientific introduces its ReActiv8 System, a neuromodulation therapy for bladder and bowel dysfunction in patients with spinal cord injuries. This technology can improve quality of life for individuals with spina bifida who experience these challenges.
List of Spina Bifida Key Companies in the Market
- Braun Melsungen AG
- Boston Scientific
- B. Fleet
- R. Bard Inc.
- Coloplast A/S
- ConvaTec Group plc
- Cook Medical
- Covidien Private Ltd.
- Dynarex Corporation
- Ethicon
- Fujifilm
- Hollister Incorporated
- KARL STORZ
- Medline Industries
- Medtronic plc
- Olympus
- Pacific Hospital Supply Co. Ltd.
- Smith & Nephew
- Stryker
- Teleflex Incorporation
- Wellspect Healthcare