Regulatory Compliance Requirements
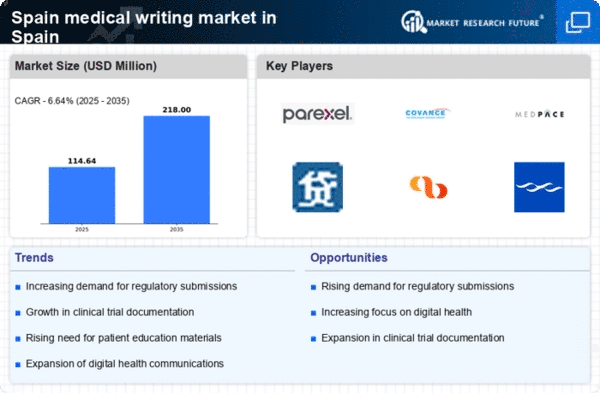
Regulatory compliance remains a critical driver for the medical writing market in Spain. The stringent guidelines set forth by agencies such as the European Medicines Agency (EMA) necessitate meticulous documentation for drug approval processes. As the regulatory landscape evolves, pharmaceutical and biotechnology companies are increasingly reliant on professional medical writers to ensure that their submissions meet all necessary standards. This reliance is likely to grow, as non-compliance can result in significant delays and financial penalties. The medical writing market is thus positioned to expand, as organizations seek to mitigate risks associated with regulatory submissions and enhance their chances of successful product approvals.
Rising Investment in Healthcare R&D
The medical writing market in Spain is experiencing a notable surge due to increased investment in healthcare research and development (R&D). In recent years, the Spanish government has allocated substantial funds to enhance medical research, which has led to a growing demand for skilled medical writers. This investment is projected to reach approximately €1.5 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 5%. As pharmaceutical companies and research institutions seek to comply with stringent regulatory requirements, the need for precise and well-structured documentation becomes paramount. Consequently, the medical writing market is likely to benefit from this trend, as organizations require expert writers to produce high-quality clinical study reports, regulatory submissions, and other essential documents.
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector in Spain is expanding rapidly, which is significantly impacting the medical writing market. With the rise of innovative therapies and biologics, there is an increasing need for comprehensive documentation to support clinical trials and regulatory submissions. The biopharmaceutical industry is expected to grow at a CAGR of 6% over the next five years, leading to a heightened demand for medical writing services. This growth necessitates the expertise of medical writers who can effectively communicate complex scientific information to regulatory bodies. As companies strive to bring new treatments to market, the medical writing market is poised to thrive, driven by the need for accurate and compliant documentation.
Increased Focus on Patient-Centric Approaches
The medical writing market in Spain is also influenced by the growing emphasis on patient-centric approaches in healthcare. As stakeholders prioritize patient engagement and outcomes, there is a corresponding need for medical writers to create materials that resonate with patients and healthcare providers alike. This shift is prompting the development of patient-friendly documents, such as informed consent forms and educational materials. The market for such services is projected to grow by approximately 4% annually, reflecting the increasing importance of clear communication in clinical research. Consequently, medical writers who can bridge the gap between complex medical information and patient understanding are becoming invaluable assets in the industry.
Technological Advancements in Medical Writing
Technological advancements are reshaping the medical writing market in Spain, as digital tools and platforms enhance the efficiency and accuracy of writing processes. The integration of artificial intelligence (AI) and machine learning is streamlining document creation, allowing medical writers to focus on higher-level tasks. This trend is expected to drive productivity in the medical writing market, with a projected increase in demand for tech-savvy writers who can leverage these tools effectively. As organizations adopt innovative solutions to improve their documentation processes, the medical writing market is likely to see a shift towards more collaborative and efficient workflows, ultimately benefiting both writers and clients.