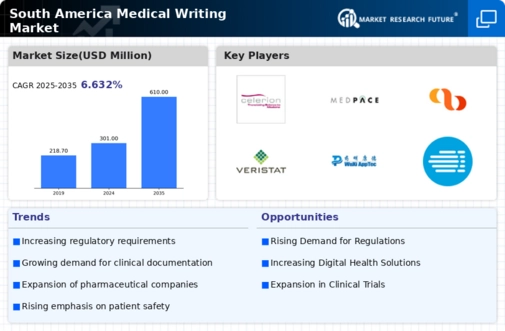
The South America Medical Writing Market is characterized by a dynamic competitive landscape influenced by various factors, including increased demand for regulatory compliance, the growth of the pharmaceutical and biotechnology sectors, and the rising complexity of clinical trials. The market features several key players who are vying for dominance through comprehensive offerings in medical writing services, including protocol writing, clinical study reports, and regulatory submissions.
Companies in this sector are focusing not only on delivering high-quality documentation but also on ensuring compliance with local and international regulations to foster trust with their clients. This scenario has cultivated an environment of strategic partnerships, mergers, and innovative approaches aimed at enhancing service delivery and broadening market reach within the region.
Parexel International holds a prominent position within the South America Medical Writing Market, leveraging its extensive experience and established reputation. The company is known for its robust capabilities in providing high-quality medical writing services tailored to meet the specific needs of the region. Its strengths include a dedicated team of expert writers knowledgeable in local regulations and standards, which enables them to produce documents with precision and clarity.
Moreover, Parexel International benefits from a strong presence in the South American market, allowing it to build lasting relationships with various stakeholders, including pharmaceutical companies and regulatory agencies. The company's commitment to excellence, coupled with an emphasis on continuous training and adaptation to evolving regulatory frameworks, positions it as a reliable partner in the region's medical writing arena.
Covance, another key player in the South American Medical Writing Market, offers a diverse range of services encompassing all stages of clinical trials. The company’s medical writing division focuses on generating high-quality regulatory documents that are essential for successful market approval processes. Covance’s strength lies in its extensive global network and resources that enable it to execute complex projects efficiently while adhering to local regulatory guidelines.
The company's proficiency is enhanced by its active involvement in mergers and acquisitions, bolstering its capabilities and expanding its service portfolio across the region. By continuously innovating and aligning its services with client needs, Covance maintains a significant foothold in the South American market, effectively addressing the growing demands of the pharmaceutical and biotechnology sectors in terms of strategic documentation and compliance services.