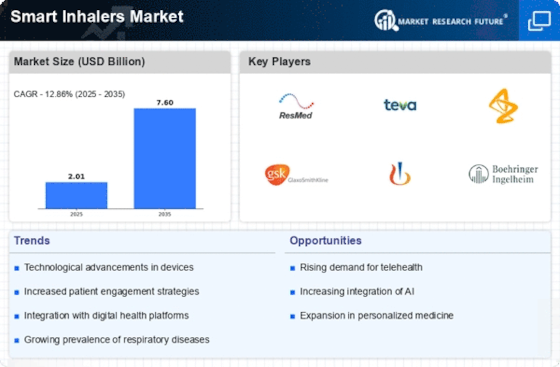
Market Trends
Key Emerging Trends in the Smart Inhalers Market
The smart inhalers market is going through remarkable changes that imply the growing scope of healthcare and technology in respiratory disease treatment. A striking trend is the rising popularity of digital healthcare solutions where smart inhalers appear in the first line. These gadgets are incorporated with sensors and connections to track inhaler usage and give instantaneous details about medication adherence and inhalation technics. This trend is especially key for patients with chronic respiratory diseases like asthma and COPD who need to adhere to their prescribed inhaler schedule in order to manage their diseases effectively.
What’s more, the smart inhalers market is more and more focusing on individualized medicine. Now, with patient-centric healthcare, smart inhalers provide the possibility of drug prescription plans that are adjusted according to individual patient needs and behavior patterns of response to treatment drugs. The incorporation of data analytics and machine learning into smart inhaler platforms can provide healthcare providers with the information about patient-specific trends in order to develop personalized and well targeted remedial efforts.
Also, the smart inhalers market has been showing co-operation between pharmaceutical companies and technology firms. These associations, however, seek to intensify the overall effectiveness of smart inhalers by complementing pharmaceutical knowledge with latest sensor technologies and data analytics. The associated rise is believed to be the acknowledgement of the interplay dynamics between the drug and the technology sectors in terms of the development and improvement of patient outcomes in respiratory care.
Also, the researchers have discovered that the inhalers will be produced with environmental sensors built in. Such sensors can measure pollution and allergen levels in the air allowing the users to understand what environmental factors may influence their respiratory health and make informed decisions. The trend coincides with the recognition of the environmental triggers of respiratory diseases and provides people with a chance to identify for themselves when they should use their inhalers by evaluating the real-time environmental data.
Another important trend is the incorporation of smart inhalers along with telemedicine and remote patient monitoring apps. Through the rise of telehealth, connected inhalers are having a positive impact on the easy monitoring of home respiratory patients. Through remote monitoring, healthcare providers are able to assess inhaler usage data in real time, provide timely interventions and hold virtual meetings to their respiratory care patients, thus enhancing the accessibility and convenience of respiratory care services.
Additionally, the market of smart inhalers is seeing revolutionary improvements in inhaler design and functions. Addressing the inefficiencies of conventional inhalers, manufacturers concentrate on creating user-friendly and ergonomic devices with improved user experience. Among these features are, but not limited to, dose reminder, inhalation guidance, and interactive interfaces, thus making smart inhalers more inclusive and user-focused for people of all ages.

















Leave a Comment