Increased Healthcare Expenditure
Rising healthcare expenditure is another significant driver for the Sleep Apnea Device Market. As countries allocate more resources to healthcare, there is a corresponding increase in spending on medical devices, including those for sleep apnea. This trend is particularly evident in developed nations, where healthcare systems are increasingly prioritizing the diagnosis and treatment of sleep disorders. According to recent data, healthcare spending is projected to grow at a compound annual growth rate of 5.4% over the next several years. This increase in funding allows for better access to sleep apnea devices, thereby expanding the market. The Sleep Apnea Device Market stands to benefit from this trend as more patients gain access to necessary treatments.
Rising Prevalence of Sleep Apnea
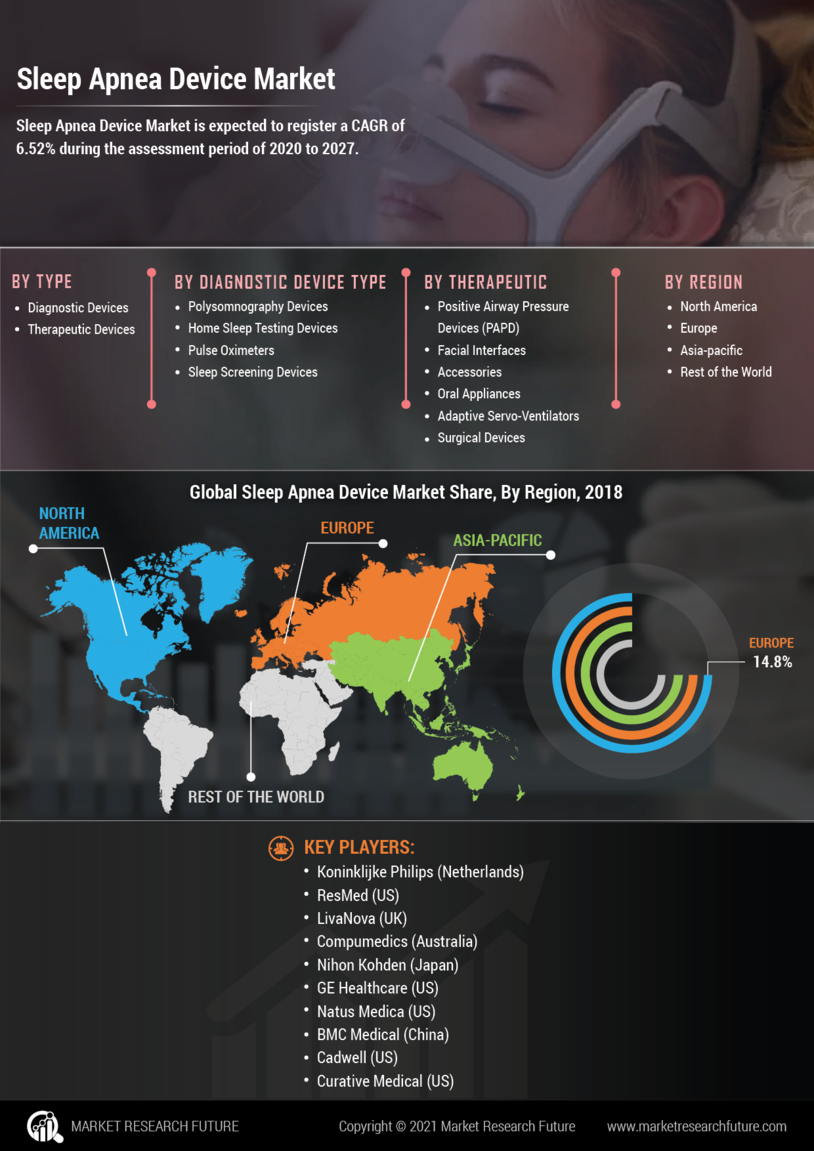
The increasing prevalence of sleep apnea is a primary driver for the Sleep Apnea Device Market. Studies indicate that approximately 22 million individuals in the United States suffer from sleep apnea, with many cases remaining undiagnosed. This growing number of patients necessitates the development and distribution of effective treatment devices. As awareness of the condition rises, healthcare providers are more likely to recommend sleep apnea devices, thereby expanding the market. Furthermore, the aging population, which is more susceptible to sleep disorders, contributes to the demand for these devices. The Sleep Apnea Device Market is thus poised for growth as more individuals seek diagnosis and treatment options.
Shift Towards Preventive Healthcare
The shift towards preventive healthcare is influencing the Sleep Apnea Device Market significantly. As healthcare systems worldwide emphasize prevention over treatment, there is a growing focus on early diagnosis and management of sleep disorders. This proactive approach encourages individuals to seek screening for sleep apnea, leading to increased demand for diagnostic devices and treatment options. Market trends indicate that preventive healthcare strategies are likely to continue gaining traction, further driving the need for sleep apnea devices. The Sleep Apnea Device Market is thus positioned to expand as healthcare providers and patients alike recognize the value of addressing sleep disorders before they escalate into more serious health issues.
Growing Awareness of Sleep Disorders
The growing awareness of sleep disorders among the general population is a vital driver for the Sleep Apnea Device Market. Educational campaigns and initiatives by healthcare organizations have led to increased recognition of the symptoms and risks associated with sleep apnea. As individuals become more informed, they are more likely to seek medical advice and treatment, resulting in higher demand for sleep apnea devices. Additionally, the media's role in highlighting the importance of sleep health contributes to this awareness. This shift in public perception is expected to drive growth in the Sleep Apnea Device Market as more patients pursue effective solutions for their sleep-related issues.
Technological Innovations in Treatment Devices
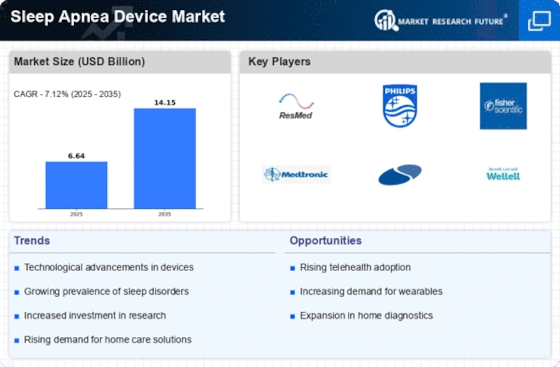
Technological advancements play a crucial role in shaping the Sleep Apnea Device Market. Innovations such as continuous positive airway pressure (CPAP) machines, oral appliances, and adaptive servo-ventilation devices have significantly improved patient compliance and comfort. The introduction of smart devices that monitor sleep patterns and provide real-time feedback enhances the user experience and encourages adherence to treatment. Market data suggests that the CPAP segment alone is expected to witness substantial growth, driven by these technological improvements. As manufacturers continue to invest in research and development, the Sleep Apnea Device Market is likely to see a surge in new product offerings, catering to diverse patient needs.