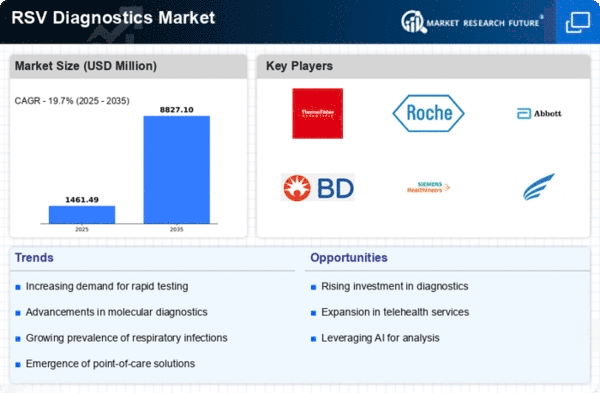
Top Industry Leaders in the RSV Diagnostics Market

BD (Becton, Dickinson and Company) receives Emergency Use Authorization (EUA) from the FDA for its BD MAX Molecular Diagnostic System combo test simultaneously detects SARS-CoV-2, influenza A+B, and RSV, offering healthcare professionals a rapid and comprehensive diagnostic tool.
Cepheid launches Xpert® Xpress RSV/Flu/Adenovirus multiplex real-time PCR test rapidly identifies RSV, influenza A and B, and adenovirus from nasopharyngeal swab specimens.
Abbott introduces ID NOW™ COVID-19/RSV/Flu Combo Test rapid molecular test delivers results in about 15 minutes, enabling quick and accurate diagnosis at the point of care.
Roche Diagnostics partners with QIAGEN to develop and commercialize a combined RSV and influenza A/B test collaboration leverages both companies' expertise in molecular diagnostics to offer a comprehensive solution for respiratory virus detection.
LumiraDX enters into a strategic partnership with GSK plc to expand access to its rapid RSV test in developing countries initiative aims to improve RSV diagnosis and management in regions with limited resources.
List of RSV Diagnostics Key Companies in the Market
- Quest Diagnostics
- Biomerieux
- Becton
- Dickinson
- and Company (BD)
- Abbott
- Hoffman-La Roche Ltd.
- Danaher Corporation
- Thermo Fisher Scientific
- Biocartis
- Luminex
- Hologic
- Fast Track Diagnostics
- Bio-Rad Laboratories Inc.
- Alere Inc.
- Quidel Corporation