-
EXECUTIVE SUMMARY
-
Market Overview
-
Key Findings
-
Market Segmentation
-
Competitive Landscape
-
Challenges and Opportunities
-
Future Outlook
-
MARKET INTRODUCTION
-
Definition
-
Scope of the study
- Research Objective
- Assumption
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
- Primary Interviews and Information Gathering Process
- Breakdown of Primary Respondents
-
Forecasting Model
-
Market Size Estimation
- Bottom-Up Approach
- Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Value chain Analysis
-
Porter's Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
COVID-19 Impact Analysis
- Market Impact Analysis
- Regional Impact
- Opportunity and Threat Analysis
-
Renal Cancer Drug Market, BY THERAPEUTIC AREAS (USD BILLION)
-
Targeted Therapy
-
Immunotherapy
-
Chemotherapy
-
Renal Cancer Drug Market, BY DRUG CLASS (USD BILLION)
-
Monoclonal Antibodies
-
Tyrosine Kinase Inhibitors
-
Checkpoint Inhibitors
-
Chemotherapeutic Agents
-
Renal Cancer Drug Market, BY ADMINISTRATION ROUTE (USD BILLION)
-
Oral
-
Intravenous
-
Subcutaneous
-
Renal Cancer Drug Market, BY END USER (USD BILLION)
-
Hospitals
-
Oncology Clinics
-
Research Institutions
-
Renal Cancer Drug Market, BY REGIONAL (USD BILLION)
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Russia
- Italy
- Spain
- Rest of Europe
-
APAC
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Indonesia
- Rest of APAC
-
South America
- Brazil
- Mexico
- Argentina
- Rest of South America
-
MEA
- GCC Countries
- South Africa
- Rest of MEA
-
COMPETITIVE LANDSCAPE
-
Overview
-
Competitive Analysis
-
Market share Analysis
-
Major Growth Strategy in the Renal Cancer Drugs Market
-
Competitive Benchmarking
-
Leading Players in Terms of Number of Developments in the Renal Cancer Drugs Market
-
Key developments and growth strategies
- New Product Launch/Service Deployment
- Merger & Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
- Sales and Operating Income
- Major Players R&D Expenditure. 2023
-
COMPANY PROFILES
-
Merck and Co
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Pfizer
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Ipsen
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Roche
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Eli Lilly
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Johnson and Johnson
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
GSK
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Novartis
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Exelixis
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Teva Pharmaceuticals
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Bayer
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
BristolMyers Squibb
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
AstraZeneca
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Amgen
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Sanofi
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
APPENDIX
-
References
-
Related Reports
-
LIST OF TABLES
-
LIST OF ASSUMPTIONS
-
NORTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
US RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
US RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
US RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
US RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
US RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
CANADA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
CANADA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
CANADA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
CANADA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
CANADA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
GERMANY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
GERMANY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
GERMANY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
GERMANY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
GERMANY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
UK RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
UK RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
UK RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
UK RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
UK RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
FRANCE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
FRANCE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
FRANCE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
FRANCE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
FRANCE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
RUSSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
RUSSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
RUSSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
RUSSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
RUSSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
ITALY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
ITALY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
ITALY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
ITALY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
ITALY RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SPAIN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
SPAIN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
SPAIN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
SPAIN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SPAIN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
CHINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
CHINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
CHINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
CHINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
CHINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
INDIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
INDIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
INDIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
INDIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
INDIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
JAPAN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
JAPAN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
JAPAN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
JAPAN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
JAPAN RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MALAYSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
MALAYSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
MALAYSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
MALAYSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MALAYSIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
THAILAND RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
THAILAND RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
THAILAND RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
THAILAND RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
THAILAND RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
INDONESIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
INDONESIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
INDONESIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
INDONESIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
INDONESIA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
REST OF APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
REST OF APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
REST OF APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF APAC RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
BRAZIL RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
BRAZIL RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
BRAZIL RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
BRAZIL RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
BRAZIL RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MEXICO RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
MEXICO RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
MEXICO RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
MEXICO RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MEXICO RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
ARGENTINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
ARGENTINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
ARGENTINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
ARGENTINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
ARGENTINA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019-2035 (USD BILLIONS)
-
REST OF MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY DRUG CLASS, 2019-2035 (USD BILLIONS)
-
REST OF MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019-2035 (USD BILLIONS)
-
REST OF MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF MEA RENAL CANCER DRUGS MARKET SIZE ESTIMATES & Renal Cancer Drug Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
PRODUCT LAUNCH/PRODUCT DEVELOPMENT/APPROVAL
-
ACQUISITION/PARTNERSHIP
-
LIST OF FIGURES
-
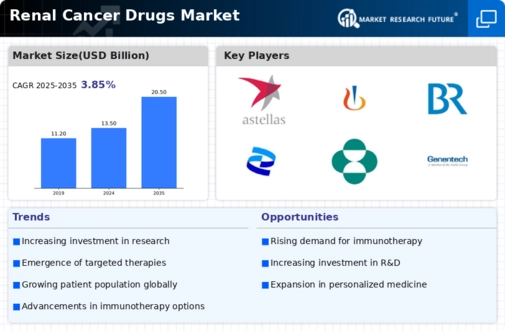
MARKET SYNOPSIS
-
NORTH AMERICA RENAL CANCER DRUGS MARKET ANALYSIS
-
US RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
US RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
US RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
US RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
US RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
CANADA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
CANADA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
CANADA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
CANADA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
CANADA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
EUROPE RENAL CANCER DRUGS MARKET ANALYSIS
-
GERMANY RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
GERMANY RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
GERMANY RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
GERMANY RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
GERMANY RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
UK RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
UK RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
UK RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
UK RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
UK RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
FRANCE RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
FRANCE RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
FRANCE RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
FRANCE RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
FRANCE RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
RUSSIA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
RUSSIA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
RUSSIA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
RUSSIA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
RUSSIA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
ITALY RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
ITALY RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
ITALY RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
ITALY RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
ITALY RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
SPAIN RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
SPAIN RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
SPAIN RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
SPAIN RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
SPAIN RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
REST OF EUROPE RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
REST OF EUROPE RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
REST OF EUROPE RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
REST OF EUROPE RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
REST OF EUROPE RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
APAC RENAL CANCER DRUGS MARKET ANALYSIS
-
CHINA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
CHINA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
CHINA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
CHINA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
CHINA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
INDIA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
INDIA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
INDIA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
INDIA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
INDIA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
JAPAN RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
JAPAN RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
JAPAN RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
JAPAN RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
JAPAN RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
SOUTH KOREA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
SOUTH KOREA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
SOUTH KOREA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
SOUTH KOREA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
SOUTH KOREA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
MALAYSIA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
MALAYSIA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
MALAYSIA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
MALAYSIA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
MALAYSIA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
THAILAND RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
THAILAND RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
THAILAND RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
THAILAND RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
THAILAND RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
INDONESIA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
INDONESIA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
INDONESIA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
INDONESIA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
INDONESIA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
REST OF APAC RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
REST OF APAC RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
REST OF APAC RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
REST OF APAC RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
REST OF APAC RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
SOUTH AMERICA RENAL CANCER DRUGS MARKET ANALYSIS
-
BRAZIL RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
BRAZIL RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
BRAZIL RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
BRAZIL RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
BRAZIL RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
MEXICO RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
MEXICO RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
MEXICO RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
MEXICO RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
MEXICO RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
ARGENTINA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
ARGENTINA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
ARGENTINA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
ARGENTINA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
ARGENTINA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
REST OF SOUTH AMERICA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
MEA RENAL CANCER DRUGS MARKET ANALYSIS
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
GCC COUNTRIES RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
SOUTH AFRICA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
REST OF MEA RENAL CANCER DRUGS MARKET ANALYSIS BY THERAPEUTIC AREAS
-
REST OF MEA RENAL CANCER DRUGS MARKET ANALYSIS BY DRUG CLASS
-
REST OF MEA RENAL CANCER DRUGS MARKET ANALYSIS BY ADMINISTRATION ROUTE
-
REST OF MEA RENAL CANCER DRUGS MARKET ANALYSIS BY END USER
-
REST OF MEA RENAL CANCER DRUGS MARKET ANALYSIS BY REGIONAL
-
KEY BUYING CRITERIA OF RENAL CANCER DRUGS MARKET
-
RESEARCH PROCESS OF MRFR
-
DRO ANALYSIS OF RENAL CANCER DRUGS MARKET
-
DRIVERS IMPACT ANALYSIS: RENAL CANCER DRUGS MARKET
-
RESTRAINTS IMPACT ANALYSIS: RENAL CANCER DRUGS MARKET
-
SUPPLY / VALUE CHAIN: RENAL CANCER DRUGS MARKET
-
Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2025 (% SHARE)
-
Renal Cancer Drug Market, BY THERAPEUTIC AREAS, 2019 TO 2035 (USD Billions)
-
Renal Cancer Drug Market, BY DRUG CLASS, 2025 (% SHARE)
-
Renal Cancer Drug Market, BY DRUG CLASS, 2019 TO 2035 (USD Billions)
-
Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2025 (% SHARE)
-
Renal Cancer Drug Market, BY ADMINISTRATION ROUTE, 2019 TO 2035 (USD Billions)
-
Renal Cancer Drug Market, BY END USER, 2025 (% SHARE)
-
Renal Cancer Drug Market, BY END USER, 2019 TO 2035 (USD Billions)
-
Renal Cancer Drug Market, BY REGIONAL, 2025 (% SHARE)
-
Renal Cancer Drug Market, BY REGIONAL, 2019 TO 2035 (USD Billions)
-
BENCHMARKING OF MAJOR COMPETITORS

















Leave a Comment