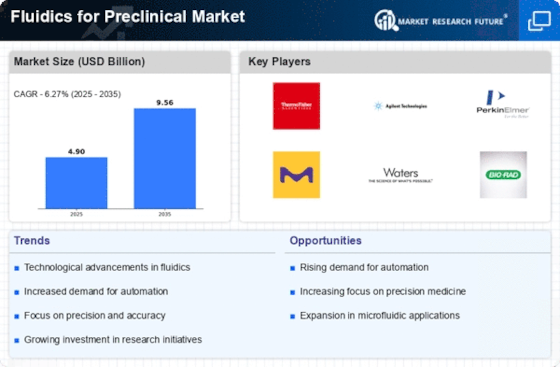
Rising Demand for Personalized Medicine
The increasing emphasis on personalized medicine is a pivotal driver for the Fluidics for Preclinical Market. As healthcare shifts towards tailored therapies, fluidic systems play a crucial role in drug development and testing. These systems enable precise control over experimental conditions, facilitating the creation of patient-specific models. The market for personalized medicine is projected to reach substantial figures, with estimates suggesting it could exceed 2 trillion USD by 2025. This trend necessitates advanced fluidic technologies that can accommodate diverse biological samples and complex assays, thereby enhancing the efficiency of preclinical studies.
Advancements in Microfluidics Technology
Technological advancements in microfluidics are significantly influencing the Fluidics for Preclinical Market. Innovations in this field allow for the manipulation of small volumes of fluids, which is essential for high-throughput screening and analysis. The microfluidics market is anticipated to grow at a compound annual growth rate of over 20% in the coming years. This growth is driven by the need for more efficient and cost-effective solutions in drug discovery and development. As researchers seek to optimize their workflows, the integration of microfluidic devices into preclinical studies is likely to become increasingly prevalent.
Growing Focus on High-Throughput Screening
The growing focus on high-throughput screening (HTS) methodologies is a notable driver for the Fluidics for Preclinical Market. HTS allows researchers to conduct thousands of experiments simultaneously, significantly reducing the time required for drug discovery. The market for HTS technologies is projected to grow substantially, with estimates indicating a potential increase to over 30 billion USD by 2026. Fluidic systems are integral to this process, as they facilitate the precise delivery and mixing of reagents, thereby enhancing the accuracy and reproducibility of results. As the demand for rapid drug development intensifies, the role of fluidics in preclinical research becomes increasingly critical.
Increased Investment in Biopharmaceutical Research
The surge in investment directed towards biopharmaceutical research is a significant driver for the Fluidics for Preclinical Market. With biopharmaceuticals accounting for a growing share of the pharmaceutical market, estimated to reach 500 billion USD by 2025, the demand for innovative fluidic systems is on the rise. These systems are essential for conducting preclinical trials that assess the efficacy and safety of new biopharmaceuticals. As funding for research and development continues to expand, fluidic technologies that enhance experimental accuracy and throughput will likely see increased adoption in preclinical settings.
Regulatory Support for Innovative Drug Development
Regulatory bodies are increasingly supporting innovative drug development processes, which serves as a key driver for the Fluidics for Preclinical Market. Initiatives aimed at expediting the approval of new therapies encourage the adoption of advanced fluidic systems that streamline preclinical testing. Regulatory frameworks are evolving to accommodate novel technologies, thereby fostering an environment conducive to innovation. This support is crucial as it not only accelerates the development timeline but also enhances the reliability of preclinical data, ultimately leading to more effective therapeutic solutions.