Government Initiatives and Funding
Government initiatives and funding aimed at addressing mental health issues, particularly PTSD, are playing a pivotal role in shaping the Post-Traumatic Stress Disorder Market. Various governments have recognized the need for comprehensive mental health strategies, leading to increased funding for research, treatment programs, and public awareness campaigns. For instance, initiatives that provide financial support for veterans suffering from PTSD have been implemented in several countries, which not only aids in treatment but also raises awareness about the disorder. This influx of funding is likely to stimulate growth in the Post-Traumatic Stress Disorder Market, as it enables the development of new therapies and enhances access to existing treatment options for affected individuals.
Advancements in Treatment Modalities
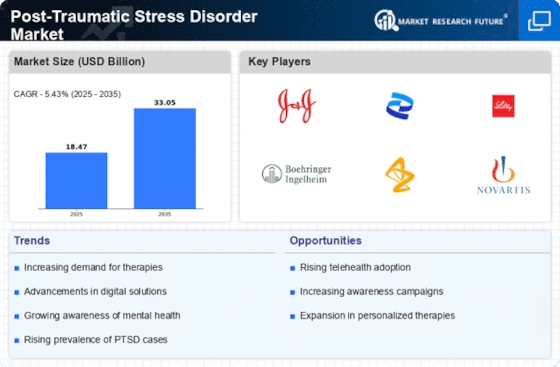
Innovations in treatment modalities for Post-Traumatic Stress Disorder are emerging as a crucial driver in the Post-Traumatic Stress Disorder Market. New therapeutic approaches, including cognitive-behavioral therapy, eye movement desensitization and reprocessing, and pharmacological advancements, are being developed and refined. The introduction of digital therapeutics and telehealth services has also transformed how patients access care, making it more convenient and accessible. Market data indicates that The Post-Traumatic Stress Disorder is projected to grow at a compound annual growth rate of around 5.5% over the next several years. This growth is indicative of the increasing investment in research and development aimed at improving treatment efficacy and patient outcomes, thereby enhancing the overall landscape of the Post-Traumatic Stress Disorder Market.
Integration of Technology in Treatment
The integration of technology in the treatment of Post-Traumatic Stress Disorder is becoming a notable driver in the Post-Traumatic Stress Disorder Market. The rise of mobile health applications, virtual reality therapy, and online support groups has transformed the way individuals access treatment and support. These technological advancements not only enhance patient engagement but also provide innovative solutions for managing symptoms. Market analysis indicates that the digital mental health market is anticipated to reach substantial figures in the coming years, reflecting the growing acceptance of technology in mental health care. This trend suggests that the Post-Traumatic Stress Disorder Market will continue to evolve, with technology playing a central role in improving treatment accessibility and effectiveness.
Growing Military and Veteran Population
The growing military and veteran population is emerging as a significant driver in the Post-Traumatic Stress Disorder Market. As more individuals return from active duty, the prevalence of PTSD among veterans has become increasingly apparent. Reports suggest that approximately 20% of veterans experience PTSD, highlighting the urgent need for effective treatment solutions. This demographic shift is likely to result in a heightened demand for specialized services tailored to the unique experiences of military personnel. Consequently, the Post-Traumatic Stress Disorder Market is expected to see an increase in the development of targeted therapies and support systems designed specifically for veterans, thereby addressing their specific needs and improving overall mental health outcomes.
Rising Awareness of Mental Health Issues
The increasing awareness surrounding mental health issues, particularly Post-Traumatic Stress Disorder (PTSD), appears to be a significant driver in the Post-Traumatic Stress Disorder Market. Campaigns aimed at educating the public about PTSD have gained momentum, leading to a greater understanding of its symptoms and effects. This heightened awareness is likely to encourage individuals to seek help, thereby increasing the demand for treatment options. According to recent statistics, approximately 7-8% of the population will experience PTSD at some point in their lives, which underscores the necessity for effective interventions. As more people recognize the importance of mental health, the Post-Traumatic Stress Disorder Market is expected to expand, with a growing number of healthcare providers offering specialized services.