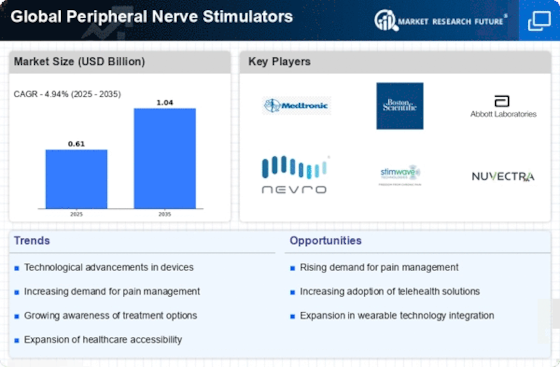
Top Industry Leaders in the Peripheral Nerve Stimulators Market
 Latest Peripheral Nerve Stimulators Companies Update
Latest Peripheral Nerve Stimulators Companies Update
Nov 2023: The US Food and Drug Administration (FDA) has approved Medtronic's Symplicity Spyralrenal Denervation (RDN) device for treating hypertension. With this permission, the company may start selling the RDN system—also called the Symplicity blood pressure procedure—immediately. The goal of the minimally invasive procedure is to apply radiofrequency radiation to the kidney-neighbor nerves, which can become hyperactive and cause elevated blood pressure. Once the patient is sedated, the physician inserts a solitary, thin tube into the kidney's artery. Once the tube is inserted, the physician stimulates the system to reduce hyperactivity in the kidney-related nerves. Although the RDN system is now only available for experimental use in Japan, China, and Canada, it has gained commercial approval in over 70 countries globally.
Apr 2023: Neuspera® Medical, a medical device business that creates implantable devices for patients with chronic conditions, announced that the FDA in the United States has approved its next-generation ultra-miniaturized Neuspera system. The system consists of a micro-implant that uses a wireless platform with an iPad-based clinician programmer and wearable transmitter to deliver neurostimulation therapy. Compared to commercially available technology, the Neuspera system uses a wireless, less invasive, and more adaptable platform to deliver peripheral nerve stimulation (PNS). It is the first PNS device to provide an ultra-minimized alternative, potentially improving patient comfort and increasing procedural flexibility.List of Peripheral Nerve Stimulators Key companies in the market
- Medtronic (Ireland)
- Braun SE (Germany)
- Boston Scientific Corporation (US)
- Curonix LLC (US)
- SPR Therapeutics (US)
- AVNS (US)
- Teleflex Incorporated (US)
- SunMed (US)
- Xavant Technology (Pty) Ltd (South Africa)
- Vygon (France)