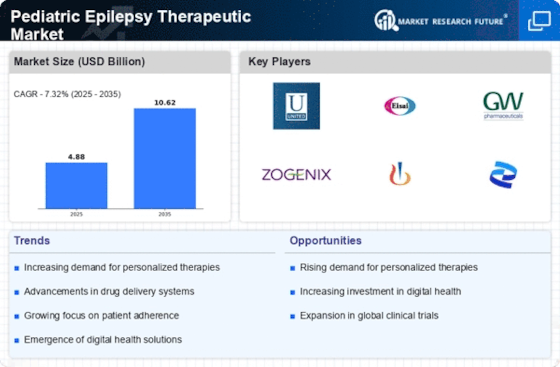
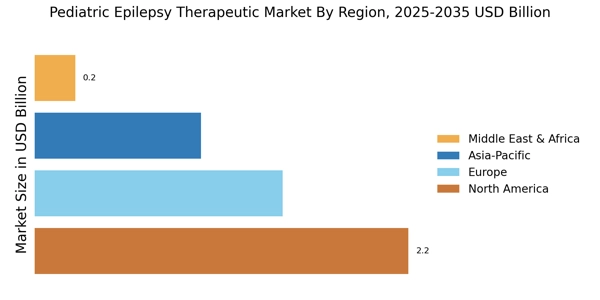
Increased Awareness and Education
Increased awareness and education regarding pediatric epilepsy are pivotal in shaping the Pediatric Epilepsy Therapeutic Market. Campaigns aimed at educating parents, educators, and healthcare professionals about the condition have led to earlier diagnosis and intervention. This heightened awareness is likely to result in a greater number of children receiving appropriate treatment, thereby expanding the market. Furthermore, educational initiatives that focus on the importance of managing epilepsy effectively can lead to improved quality of life for affected children. As more stakeholders become informed about the complexities of pediatric epilepsy, the demand for specialized therapeutic options is expected to rise.
Innovations in Treatment Modalities
Innovative treatment modalities are significantly influencing the Pediatric Epilepsy Therapeutic Market. The introduction of novel antiepileptic drugs (AEDs) and non-pharmacological interventions, such as ketogenic diets and neurostimulation devices, has broadened the therapeutic landscape. Recent advancements in drug formulation and delivery systems have improved the efficacy and safety profiles of these treatments. For instance, the development of liquid formulations has made it easier to administer medications to children, enhancing adherence. As these innovations continue to emerge, they are expected to drive growth in the Pediatric Epilepsy Therapeutic Market, providing new hope for families affected by epilepsy.
Rising Investment in Pediatric Research
Rising investment in pediatric research is significantly impacting the Pediatric Epilepsy Therapeutic Market. Increased funding from both public and private sectors is being directed towards understanding the underlying mechanisms of epilepsy in children and developing targeted therapies. This financial support is fostering collaborations between academic institutions, pharmaceutical companies, and healthcare organizations, leading to accelerated research and development efforts. As more resources are allocated to pediatric epilepsy research, the market is expected to witness a surge in innovative therapeutic options. This trend not only enhances the understanding of pediatric epilepsy but also drives the development of effective treatments tailored to the needs of young patients.
Growing Prevalence of Pediatric Epilepsy
The rising incidence of pediatric epilepsy is a primary driver for the Pediatric Epilepsy Therapeutic Market. Recent estimates indicate that approximately 1 in 100 children are diagnosed with epilepsy, leading to an increased demand for effective therapeutic options. This growing prevalence necessitates the development of innovative treatments tailored specifically for the pediatric population. As awareness of epilepsy increases among healthcare providers and parents, the market is likely to expand further. The need for specialized therapies that address the unique challenges faced by children with epilepsy is becoming increasingly apparent, thereby propelling the Pediatric Epilepsy Therapeutic Market forward.
Regulatory Support for Pediatric Drug Development
Regulatory support for pediatric drug development is a crucial factor driving the Pediatric Epilepsy Therapeutic Market. Regulatory agencies have implemented guidelines and incentives to encourage the development of medications specifically for children. Initiatives such as the Pediatric Research Equity Act (PREA) and the Best Pharmaceuticals for Children Act (BPCA) promote research and development in this area. These regulations not only facilitate the approval of new therapies but also ensure that existing treatments are evaluated for safety and efficacy in pediatric populations. As a result, the Pediatric Epilepsy Therapeutic Market is likely to benefit from a more robust pipeline of pediatric-specific therapies.